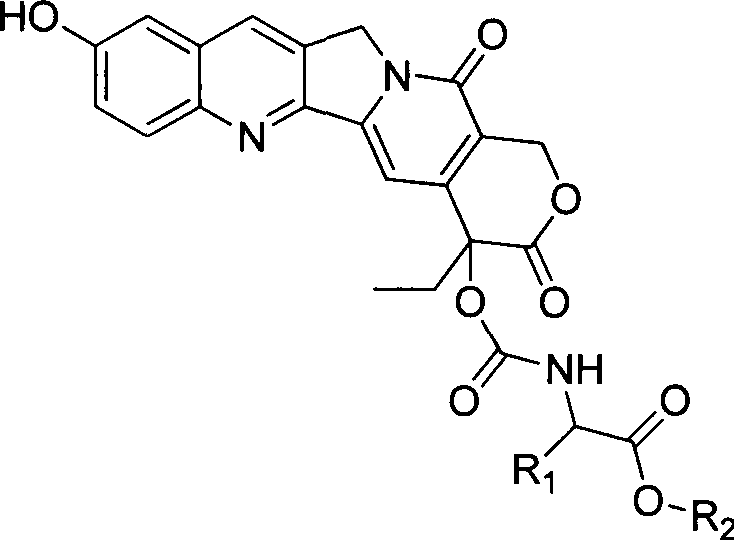
Novel 10-hydroxy camptothecin site-20 derivative preparation method, and application of 10-hydroxy camptothecin site 20 derivative in anti-tumor drugs
A technology of hydroxycamptothecin and derivatives, applied in the field of new compound preparation, can solve the problems of low antitumor activity and high toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
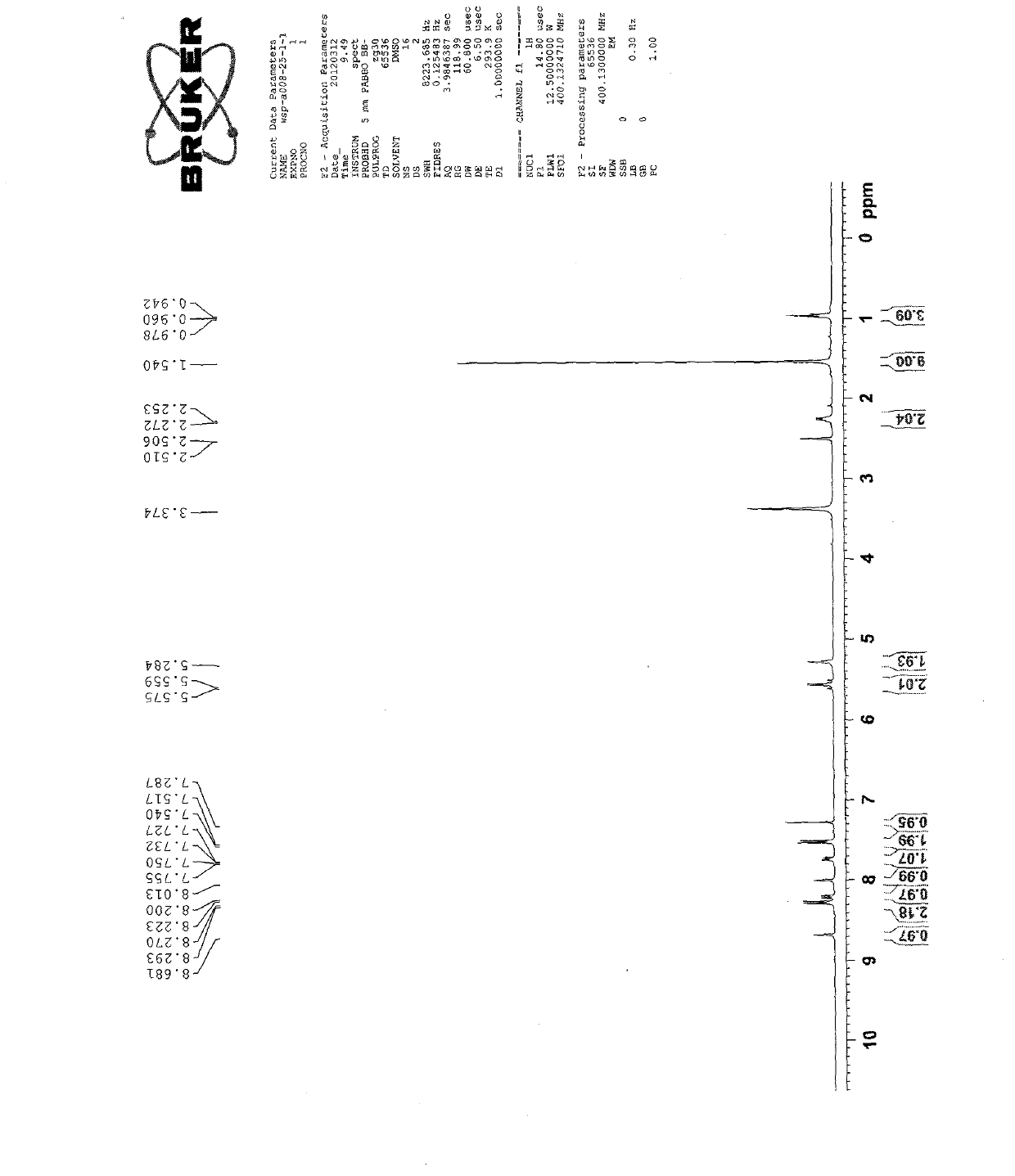
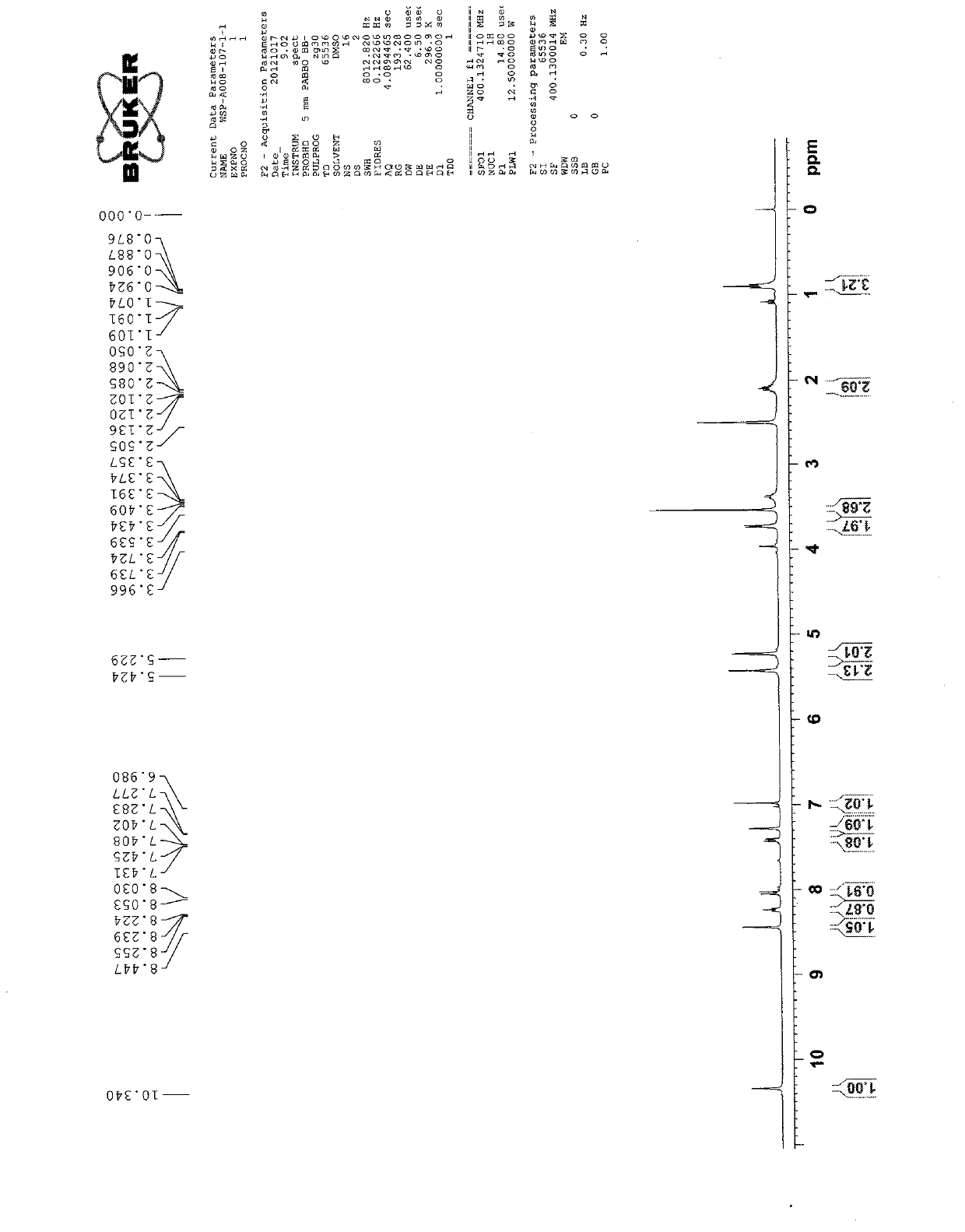
[0039] synthetic route
[0040]
[0041] Description 1
[0042] 10-tert-butoxycarbonyl-camptothecin
[0043] 10-Hydroxycamptothecin (1g, 2.74mmol) was added to a 100mL round-bottomed flask, then DMF (28mL) was added to dissolve, and then di-tert-butyl dicarbonate (1.2g, 5.5mmol) was added after fully dissolved, and stirred After 5 minutes, pyridine (10 mL) was added, the bottle was plugged, and the reaction was placed in a dark place for 24 hours. After the reaction is over, pour the reaction solution into a 250mL separatory funnel, add 90mL of dichloromethane to dissolve, then add 150mL of water each time for extraction, and extract three times; then add 36mL of 1mol / L hydrochloric acid and 114mL of water for extraction, and extract three times in the same way ; Finally, extract it once with 150mL saturated saline. Anhydrous NaSO for organic phase 4 Dry, spin off the solvent under reduced pressure, dichloromethane:methanol=120:1, 200-300 mesh silica gel column purific...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com