Method of preparing high-purity heparin sodium
A heparin sodium, high-purity technology, applied in the biological field, can solve the problems of complicated operation, time-consuming and labor-consuming, low yield, etc., and achieve the effect of simple and easy method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
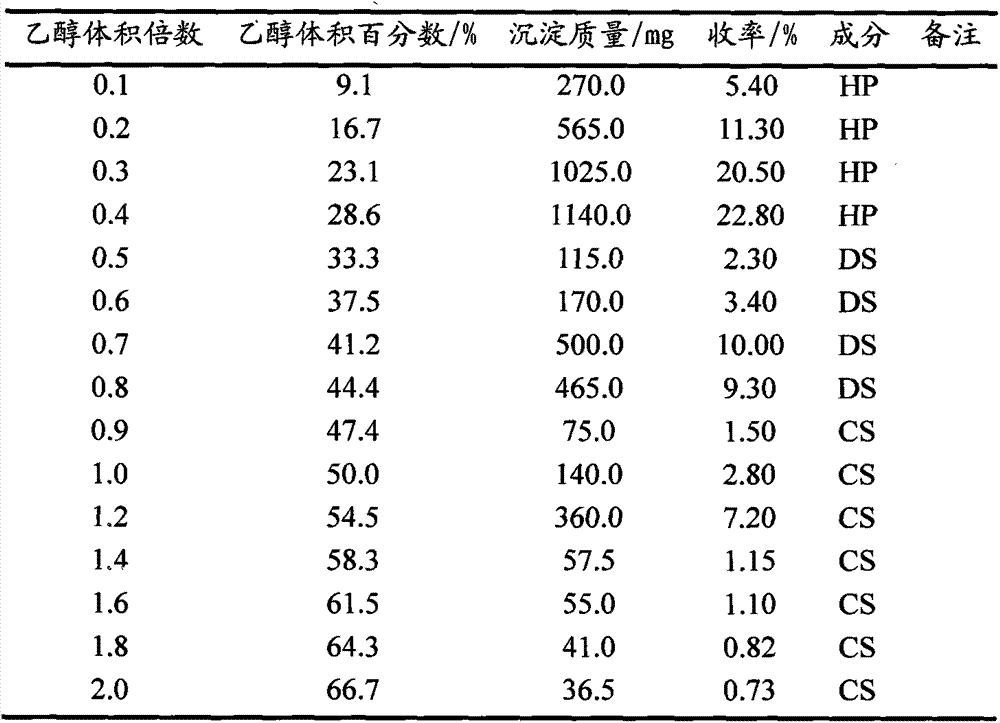
[0019] (1) Weigh 5g of heparin industrial crude product (containing different amounts of DS, CS impurities);
[0020] (2) Add 2% salt water and dissolve evenly;
[0021] (3) adding an organic solvent to the solution with a volume multiple of 9.1%-28.6% (Volume);
[0022] (4) Oscillate evenly, and stand at room temperature for 2 hours;
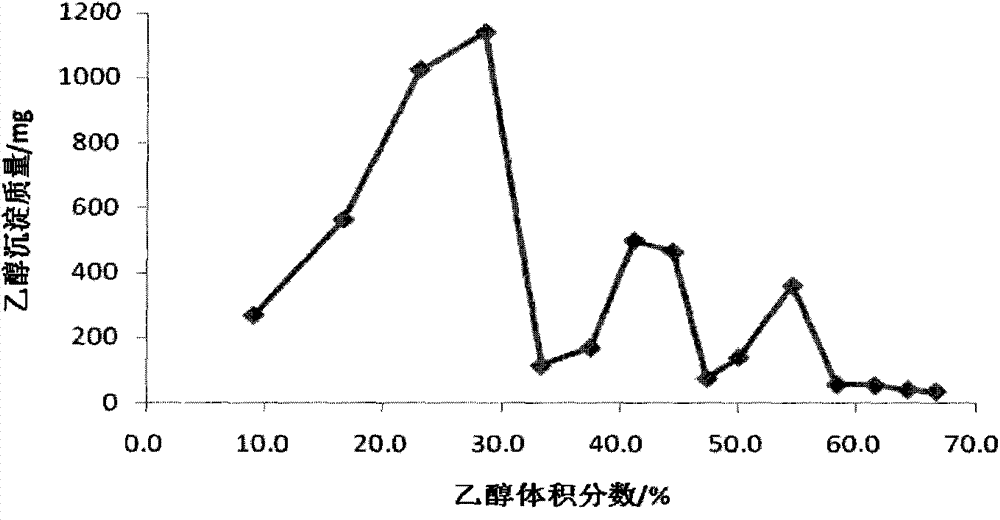
[0023] (5) Centrifuge to collect the precipitate, then adjust the volume multiple of the supernatant to 33.3%-44.4% of the organic solvent, oscillate evenly, and store at room temperature;
[0024] (6) Centrifuge to collect the precipitate, adjust the volume multiple of the supernatant to 47.4%-66.7%, centrifuge, collect the precipitate, dry each part of the precipitate, and weigh.
[0025] (7) Table 1 shows the experimental results of ethanol fractional alcohol precipitation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com