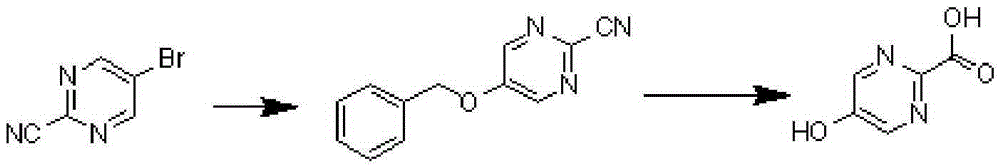
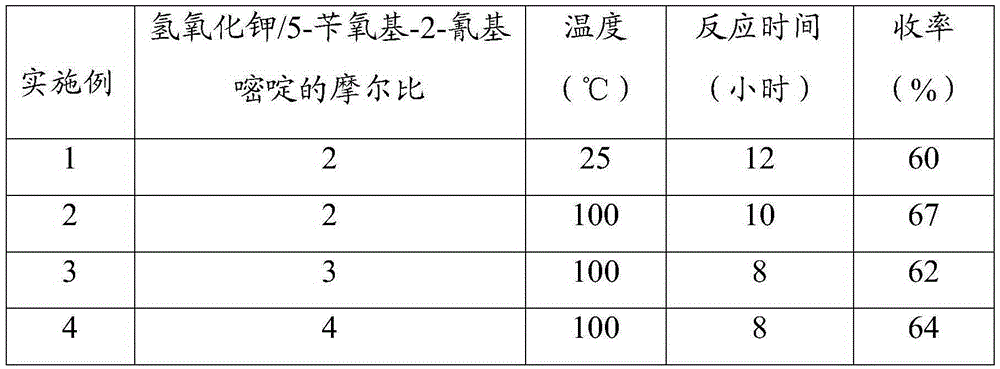
A kind of synthetic method of 5-hydroxypyrimidine-2-carboxylic acid
A synthesis method and technology of hydroxypyrimidine are applied in the field of synthesis of 5-hydroxypyrimidine-2-carboxylic acid, and achieve the effects of easy to enlarge production, high yield and few reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0022] The technical solution of the present invention is illustrated below through specific examples. The raw materials and reagents used in the present invention are all commercially available.
[0023] Step 1, Synthesis of 5-benzyloxy-2-cyanopyrimidine
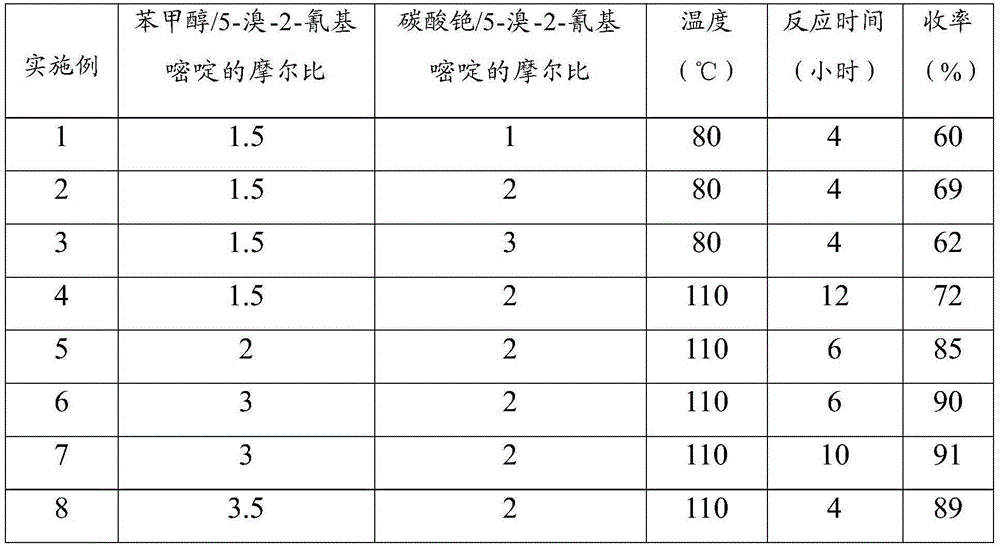
[0024] 5-Bromo-2-cyanopyrimidine (10g, 54mmol) and benzyl alcohol (17.5g, 162mmol) were dissolved in 100ml of toluene, respectively, and then cesium carbonate (35g, 108mmol), cuprous iodide (1g, 5.4 mmol), 1,10-phenanthroline (2g, 11.34mmol) (cuprous iodide and 1,10-phenanthroline added here are used as catalysts, which are the minimum amount, and the amount added is 5-bromo-2- 10% and 20% of cyanopyrimidine). After the reaction solution was stirred and reacted at 110° C. for 4 hours, TLC detected that the reaction was complete. The reaction solution was cooled to room temperature (20-25°C), concentrated and purified by column chromatography to obtain 10.3 g of 5-benzyloxy-2-cyanopyrimidine as a white solid, with a yield...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com