Application of human albumin in preparing drug for treating subarachnoid hemorrhage
A human albumin, subarachnoid technology, applied in drug combination, peptide/protein composition, pharmaceutical formulation, etc., can solve the problems of rupture of aneurysm, increase of intracranial pressure, increase of cardiac load, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] 1 Materials and methods
[0022] 1.1 Reagents and instruments
[0023] 20% human serum albumin (German Biotest), 10% chloral hydrate, Evans blue (Sigma-aldrich), formamide, water bath, oven, microplate reader (Biotech), etc.
[0024] 1.2 Experimental animals
[0025] Adult healthy male Sprague-Dawley (SD) rats, 300-350g, were provided by the Animal Experiment Center of Nanjing General Hospital of Nanjing Military Region, and were reared in a single cage for 3 days before operation to avoid the influence of non-specific factors, and were kept at room temperature 20±2°C. 12-hour light / 12-hour dark environment, free to eat and drink.
[0026] 1.3 Experimental method
[0027] 1.3.1 Animal grouping
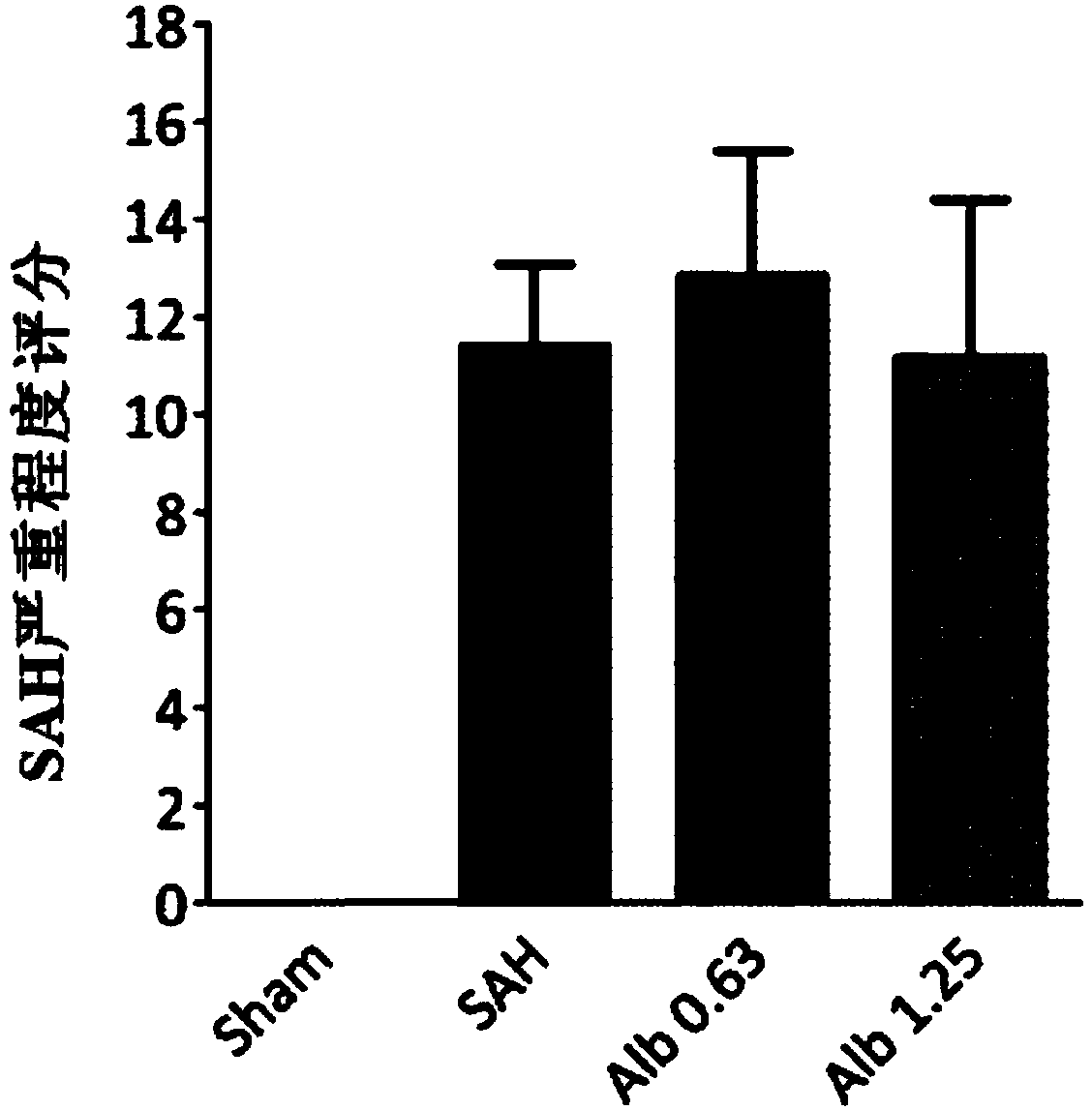
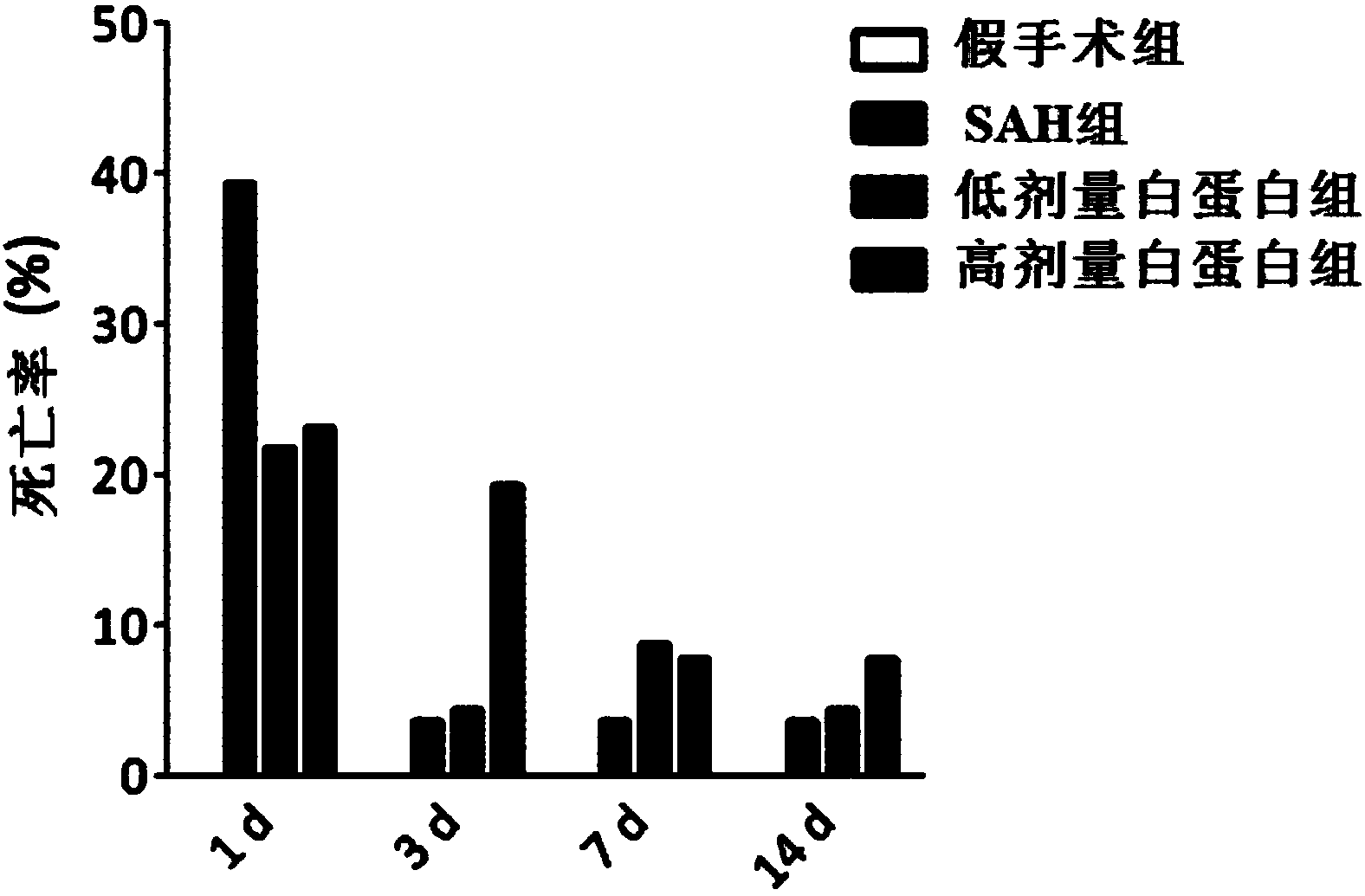
[0028] SD rats were randomly divided into sham operation group, SAH group, low-dose albumin group (0.63g / kg), high-dose albumin group (1.25g / kg).
[0029] (1) Sham operation group: the thread plug was inserted into the brain, but the blood vessel was not punctured;
[0030...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com