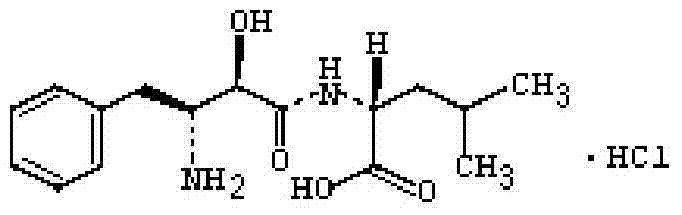
A kind of ubenimex hydrochloride compound
A technology of urbenex hydrochloride and compound, which is applied in the separation/purification of carboxylic acid amide, organic chemistry, drug combination, etc., to achieve the effect of good chemical stability and improved safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The preparation of embodiment 1 ubenimex hydrochloride crystal form
[0029] preparation:
[0030] Add 0.5 g of ubenimex hydrochloride to 22 mL of a mixed solvent of isopropyl acetate: dimethylformamide with a volume ratio of 10:1, heat slightly to dissolve, stir for 1.5 h, filter, and store in a static state. Three days later, the solvent was completely evaporated, and the solid was crushed, left to dry at room temperature for 6 hours, and samples were collected to obtain 0.45 g of crystalline form of ubenimex hydrochloride.
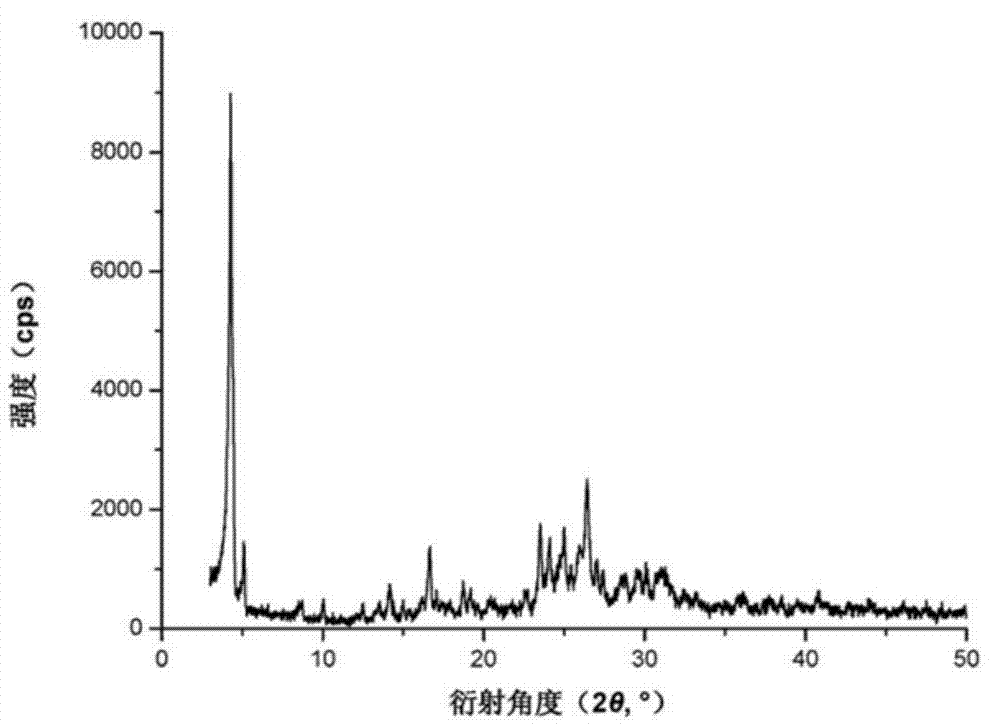
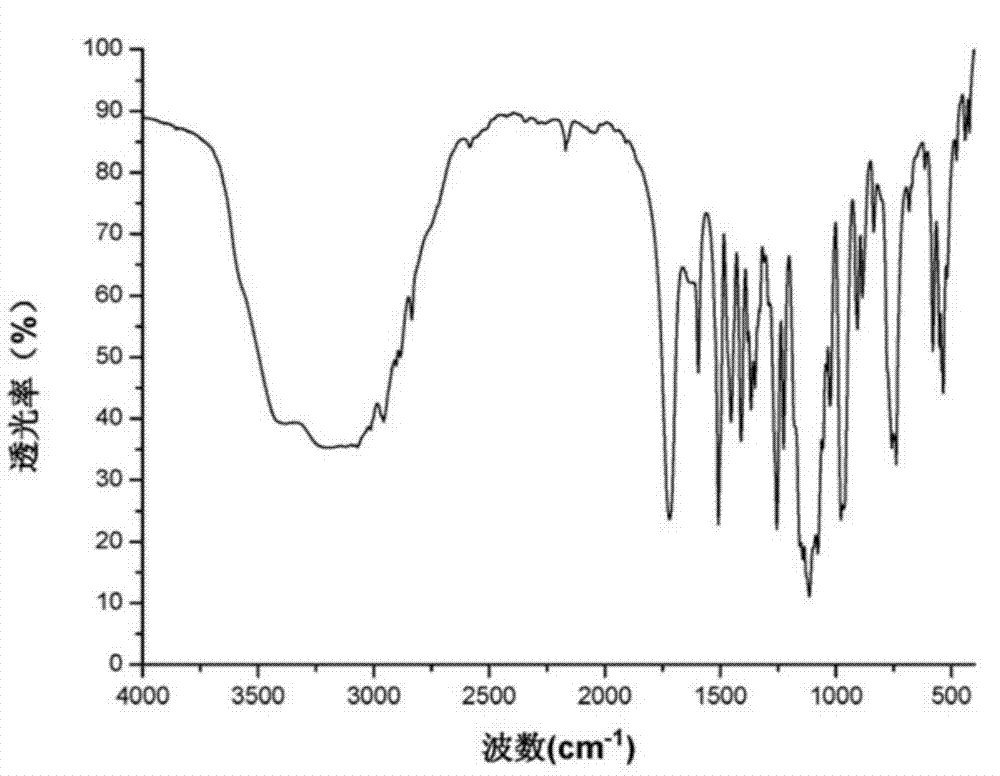
[0031] Measured under the above-mentioned instrument and condition, obtain as figure 1 with figure 2 The shown XRD-powder diffraction pattern and infrared spectrum.
Embodiment 2
[0032] The preparation of embodiment 2 ubenimex hydrochloride crystal form
[0033] Add 1.0 g of ubenimex hydrochloride to 30 mL of a mixed solvent of isopropyl acetate: dimethylformamide with a volume ratio of 14:1, heat slightly to dissolve, stir for 1.5 h, filter, and keep it standing for three After a few days, the solvent was completely evaporated, and the solid was crushed, left to dry at room temperature for 5 hours, and samples were collected to obtain 0.88 g of crystalline form of Ubenimex hydrochloride.
[0034] Carry out the mensuration as embodiment 1, obtained result is consistent with embodiment 1.
Embodiment 3
[0035] The preparation of embodiment 3 ubenimex hydrochloride crystal form
[0036] Add 1.0 g of ubenimex hydrochloride to 40 mL of a mixed solvent of isopropyl acetate: dimethylformamide with a volume ratio of 19:1, heat slightly to dissolve, stir for 2 hours, filter, and keep it standing for three days. The solvent was completely evaporated, the solid was crushed, and left to dry at room temperature for 6 hours, and the sample was collected to obtain 0.93 g of crystalline form of Ubenimex hydrochloride.
[0037] Carry out the mensuration as embodiment 1, obtained result is consistent with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com