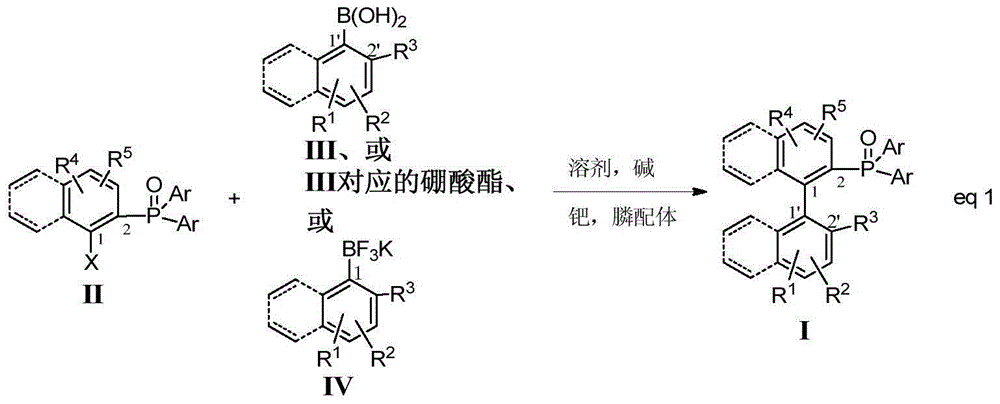
Preparation method of axially chiral biaryl phosphine oxide and axially chiral biaryl phosphine
A biaryl and axial chirality technology, which is applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc., can solve the problems of many synthesis steps, large dosage and high cost, and avoid The effect of many steps, simple operation and high synthesis efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1: Compound preparation of
[0021]
[0022] Under nitrogen protection, 103mg (0.25mmol) (1-bromo-2-naphthyl)-diphenylphosphine oxide, 68mg (0.5mmol) 2-methylphenylboronic acid, 159mg (0.75mmol) K 3 PO 4 , 5.77mg (0.012mmol) Ligand L1, 4.58mg (0.005mmol) Pd 2 (dab) 3 Add to reaction tube. 3mL of anhydrous and oxygen-free toluene was used as solvent, and reacted at 100°C for 72 hours. The reaction system was filtered to remove insoluble matter, concentrated, and the crude product was separated and purified by a silica gel column to obtain a light yellow solid (racemate). Yield: 80%. Enantioselectivity 67%ee (HPLC, Chiralcel OD-H column, 25°C, n-hexane:isopropanol=90:10, 0.5mL / min, 230nm, t R =13.71min, 15.62min). [α] 25 D -0.8 (c=5.0, CHCl 3 ), ESI-MS: 419.3[M+H] + .
[0023] 1 H NMR (300MHz, CDCl 3 )δ7.88(dd, J=8.6,2.9Hz,2H),7.72(dd,J=11.5,8.5Hz,1H),7.58-7.20(m,13H),7.14-7.09(m,1H),6.92 (d,J=7.4Hz,1H),6.89(d,J=7.1Hz,1H),6.83(d,J=7.7Hz,1H),1.6...
Embodiment 5
[0041] Example 5: Compounds preparation of
[0042]
[0043] Under nitrogen protection, 47.6mg (0.1mmol) (1-trifluoromethylsulfonate-2-naphthyl)-diphenylphosphine oxide, 40.0mg (0.2mmol) 2-biphenylboronic acid, 64mg ( 0.3mmol) K 3 PO 4 , 3.6mg (0.0048mmol) Ligand L2, 1.8mg (0.002mmol) Pd(OAc) 2 Add to reaction tube. 2mL of anhydrous and oxygen-free tetrahydrofuran was used as solvent, and reacted at 70°C for 24-120 hours. The reaction system was filtered to remove insoluble matter, concentrated, and the crude product was separated and purified by a silica gel column to obtain a light yellow solid. Yield: 46%. Enantioselectivity 65%ee (HPLC, Chiralcel OD-H column, 25°C, n-hexane:isopropanol=75:25, 1.0mL / min, 230nm, t R =7.35min, 10.42min). [α] 25 D -14.8 (c=1.0, CHCl 3 ), ESI-MS: 503.3[M+Na] + .
[0044] 1 H NMR (300MHz, CDCl 3 )δ7.76(d,J=8.4Hz,1H),7.71(d,J=8.17Hz,1H),7.32-7.50(m,15H),7.12-7.19(m,4H),686-6.97(m ,4H)ppm;
[0045] 13 C NMR (75MHz, CDCl 3 )δ...
Embodiment 6
[0047] Example 6: Compounds preparation of
[0048] Method steps are the same as in Example 5, yield: 50%, enantioselectivity 25%ee (high performance liquid chromatography, Chiralcel OD-H column, 25°C, n-hexane:isopropanol=75:25, 1.0mL / min , 230nm, t R =17.35min, 20.46min). [α] 25 D -8.8 (c=1.0, CHCl 3 ), ESI-MS: 503.3[M+Na] + .
[0049] 1 H NMR (300MHz, CDCl 3 )δ6.73-6.80(m,2H),6.84-6.87(m,4H),6.95-6.99(m,4H),7.04-7.10(m,1H),7.18-7.39(m,8H),7.49- 7.55(m,1H),7.60-7.69(m,5H)ppm;
[0050] 31 P NMR (121MHz, CDCl 3 )δ28.83ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com