Nitrogen heterocyclic butanone as well as synthesis method and application thereof
A technology of azetidinone and synthesis method, applied in the direction of organic chemistry, etc., can solve the problems of unstable reaction, affecting yield, low yield, etc., and achieve the effects of high reaction yield, reduced emissions, and reduced costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
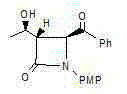
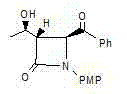
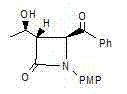
[0037] An azetidinone, the chemical name is: (3S,4S)-3-[(1R)-hydroxyethyl]-4-phenylacetyl-1-p-methoxyphenyl-2-azacyclic ring Butanone (compound Ⅰ), the structure is as follows,
[0038]
[0039] Compound I.
[0040] The synthesis method comprises the following steps: (1) Synthesis of (2S,3R)-2-amino-3-hydroxyl-N-(4-methoxyphenyl)butylamine (compound II): ethyl acetate is a solvent, L-threonine reacts with p-aminoanisole under the action of CDI to generate compound Ⅱ;
[0041] In a dry and clean reaction bottle, add 540 g of ethyl acetate under temperature control at 25°C, turn on electric stirring and add 146 g of N,N-carbonyldiimidazole, add 100 g (0.84 mol) of L-threonine in batches, and add in ten batches, After adding one batch, add the next batch when there are no more bubbles in the system, then keep stirring at 25°C for 1 h under nitrogen protection, add 98.5 g (0.8 mol) of p-aminoanisole, raise the temperature to 45°C, and Insulate and stir the reaction for 3-5 h...
Embodiment 2
[0049] A kind of azetidinone, structure is the same as embodiment 1.
[0050] The synthesis method comprises the following steps: (1) Synthesis of (2S,3R)-2-amino-3-hydroxyl-N-(4-methoxyphenyl)butylamine (compound II): ethyl acetate is a solvent, L-threonine reacts with p-aminoanisole under the action of CDI to generate compound Ⅱ;
[0051] In a dry and clean reaction bottle, add 540 g of ethyl acetate under temperature control at 27°C, turn on electric stirring and add 146 g of N,N-carbonyldiimidazole, add 100 g (0.84 mol) of L-threonine in batches, and add in ten batches, After adding one batch, add the next batch when there are no more bubbles in the system, then keep stirring at 25°C for 1 h under nitrogen protection, add 98.5 g (0.8 mol) of p-aminoanisole, raise the temperature to 55°C, and Insulate and stir the reaction for 3-5 h, monitor the reaction to the end point by TLC, and wash the organic phase with 85 ml 2 mol / L hydrochloric acid, 230 ml 10% NaHCO 3 The aqueou...
Embodiment 3
[0059] A kind of azetidinone, structure is the same as embodiment 1.
[0060] The synthesis method comprises the following steps: (1) Synthesis of (2S,3R)-2-amino-3-hydroxyl-N-(4-methoxyphenyl)butylamine (compound II): ethyl acetate is a solvent, L-threonine reacts with p-aminoanisole under the action of CDI to generate compound Ⅱ;
[0061] In a dry and clean reaction bottle, add 540 g of ethyl acetate under temperature control at 23°C, turn on electric stirring and add 146 g of N,N-carbonyldiimidazole, add 100 g (0.84 mol) of L-threonine in batches, and add in ten batches, After adding one batch, add the next batch when there are no more bubbles in the system, then keep stirring at 25°C for 1 h under nitrogen protection, add 98.5 g (0.8 mol) of p-aminoanisole, raise the temperature to 45°C, and Insulate and stir the reaction for 3-5 h, monitor the reaction to the end point by TLC, and wash the organic phase with 85 ml 2 mol / L hydrochloric acid, 230 ml 10% NaHCO 3 The aqueou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com