Derived peptide IR2 of pig-derived antibacterial peptide as well as preparation method and application thereof
A technology of derivatized peptides and antimicrobial peptides, applied in the field of porcine-derived antimicrobial peptide IR2 and its preparation, can solve the problems of unsatisfactory antibacterial activity and high cytotoxicity of natural antimicrobial peptides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Design of Antimicrobial Peptides
[0018] The amino acid sequence of porcine antimicrobial peptide PG-1 is:
[0019] Arg Gly Gly Arg Leu Cys Tyr Cys Arg Arg Arg Phe Cys Val Cys Val Gly Arg-NH 2
[0020] 1 5 10 15 18
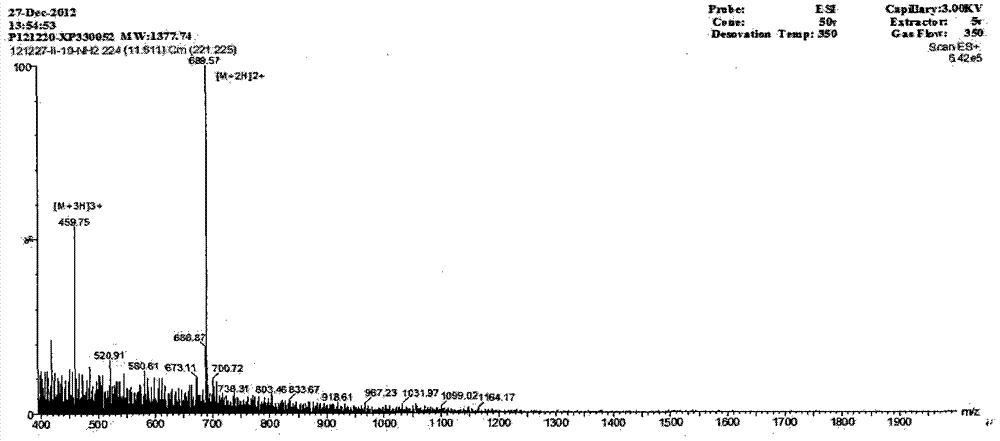
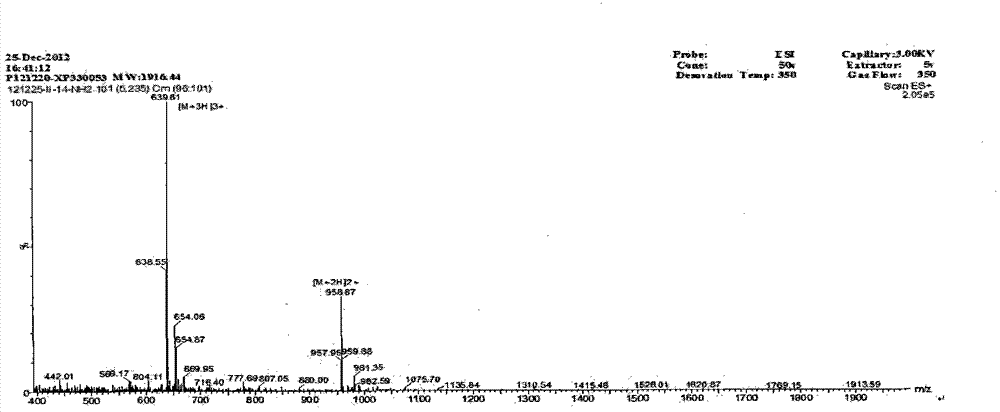
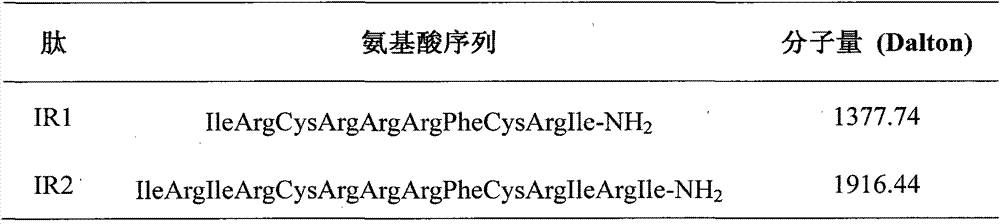
[0021] By intercepting the amino acid sequence CRRRFC (8-13 amino acids) of the β-turn position of PG-1, the charged arginine R and the hydrophobic amino acid I are selected to extend the sheets at both ends of the PG-1 turn position. By intercepting the amino acid sequence of the PG-1β-turn, and connecting the two ends of the turn with IR or RI respectively, the IR series peptides were designed: (IR) n H(RI) n -NH 2 , (n=1, 2; H is the β-turn amino acid sequence CRRRFC of PG-1), when n=1, the derived peptide is named IR1; when n=2, the derived peptide is named IR2. The amino acid sequences of the derived peptides are shown in Table 1.
[0022] Table 1 Amino Acid Sequence of Derived Peptides
[0023]
[0024] The charge numbers of IR1 and IR2 a...
Embodiment 2
[0026] Synthesis of Two Antimicrobial Peptides IR1 and IR2 by Solid Phase Chemical Synthesis
[0027] 1. The preparation of antimicrobial peptides is carried out one by one from the C-terminal to the N-terminal, and is completed by a peptide synthesizer. First, Fmoc-X (X is the first amino acid at the C-terminal of each antimicrobial peptide) is inserted into Wang resin, and then the Fmoc group is removed to obtain X-Wang resin; then Fmoc-Y-Trt-OH (9 -Fmoxy-trimethyl-Y, Y is the second amino acid at the C-terminus of each antimicrobial peptide); according to this procedure, it is synthesized from the C-terminus to the N-terminus until the synthesis is completed, and the side of the Fmoc group is removed chain protection resin;
[0028] 2. Add a cleavage reagent to the peptide resin obtained above, react for 2 hours at 20°C in the dark, filter; wash the precipitate with TFA (trifluoroacetic acid), mix the washing liquid with the above filtrate, concentrate with a rotary evapor...
Embodiment 3
[0031] Embodiment 3: the mensuration of antimicrobial peptide antibacterial activity
[0032] 1. Determination of antibacterial activity: Prepare the peptide as a storage solution for use. The minimum inhibitory concentrations of several antimicrobial peptides were determined by the broth microdilution method. Using 0.01% acetic acid (containing 0.2% BSA) as the diluent, a series of gradient antimicrobial peptide solutions were sequentially prepared using the double dilution method. Take 100 μL of the above solution and place it in a 96-well cell culture plate, then add an equal volume of the bacteria solution to be tested (~10 5 individual / mL) in each well. Positive controls (containing bacterial fluid but not antimicrobial peptides) and negative controls (neither bacterial fluid nor peptides) were set up. Incubate at a constant temperature of 37°C for 20 hours, and the minimum inhibitory concentration is the one where no turbidity is seen at the bottom of the well with th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com