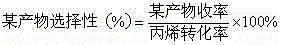Acrolein catalyst and preparation method thereof
A catalyst, acrolein technology, applied in the preparation of carbon-based compounds, chemical instruments and methods, preparation of organic compounds, etc., can solve problems such as the reduction of propylene conversion rate and acrolein yield, and achieve delaying performance decline and good technical effect. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] 1000 g (NH 4 ) 6 Mo 7 o 24 4H 2 O (i.e. ammonium heptamolybdate) was added to 1000 grams of warm water at 70°C, stirred to dissolve it completely, and 2.88 grams of KNO 3 and 5.49 grams of CsNO 3 To obtain material 1, add 573.9 grams of silica sol with a concentration of 40 wt% to material 1 to prepare material 2.
[0038] 396.9 g Fe(NO 3 ) 3 9H 2 O was added to 150 g of 70°C hot water, stirred and dissolved, and then 229.0 g of Bi(NO 3 ) 3 ·5H 2 O, 480.8 g Co(NO 3 ) 2 ·6H 2 O, 452.1 g Ni(NO 3 ) 2 ·6H 2 O, 150.5 g of 50 wt % Mn(NO 3 ) 2 Aqueous solution, 19.6 g La(NO 3 ) 3 ·3H 2 O and 41.7 g Sb 2 o 3 Stir to make material 3.
[0039]Add material 3 to material 2 under rapid stirring to form a catalyst slurry, and stir and age at 65°C for 2 hours. The slurry is sprayed and granulated and calcined at 350°C for 2 hours to obtain a powdery catalyst precursor (spray Conditions: air inlet temperature 345°C, air outlet temperature 125°C, atomization dis...
Embodiment 2
[0055] 1000 g (NH 4 ) 6 Mo 7 o 24 4H 2 O was added to 1000 grams of warm water at 70°C, stirred to dissolve it completely, and 2.88 grams of KNO was added 3 and 5.49 grams of CsNO 3 To obtain material 1, add 573.9 grams of silica sol with a concentration of 40 wt% to material 1 to prepare material 2.
[0056] 396.9 g Fe(NO 3 ) 3 9H 2 O was added to 150 g of 70°C hot water, stirred and dissolved, and then 229.0 g of Bi(NO 3 ) 3 ·5H 2 O, 480.8 g Co(NO 3 ) 2 ·6H 2 O, 452.1 g Ni(NO 3 ) 2 ·6H 2 O, 150.5 g of 50 wt % Mn(NO 3 ) 2 Aqueous solution, 19.6 g La(NO 3 ) 3 ·3H 2 O and 41.7 g Sb 2 o 3 Stir to make material 3.
[0057] Add material 3 to material 2 under rapid stirring to form a catalyst slurry, and stir and age at 65°C for 2 hours. The slurry is sprayed and granulated and calcined at 350°C for 2 hours to obtain a powdery catalyst precursor (spray Conditions: air inlet temperature 345°C, air outlet temperature 125°C, atomization disc rotation speed 220...
Embodiment 3
[0060] 1000 g (NH 4 ) 6 Mo 7 o 24 4H 2 O was added to 1000 grams of warm water at 70°C, stirred to dissolve it completely, and 2.88 grams of KNO was added 3 and 5.49 grams of CsNO 3 To obtain material 1, add 573.9 grams of silica sol with a concentration of 40 wt% to material 1 to prepare material 2.
[0061] 396.9 g Fe(NO 3 ) 3 9H 2 O was added to 150 g of 70°C hot water, stirred and dissolved, and then 229.0 g of Bi(NO 3 ) 3 ·5H 2 O, 480.8 g Co(NO 3 ) 2 ·6H 2 O, 452.1 g Ni(NO 3 ) 2 ·6H 2 O, 150.5 g of 50 wt % Mn(NO 3 ) 2 Aqueous solution, 19.6 g La(NO 3 ) 3 ·3H 2 O and 41.7 g Sb 2 o 3 Stir to make material 3.
[0062] Add material 3 to material 2 under rapid stirring to form a catalyst slurry, and stir and age at 65°C for 2 hours. The slurry is sprayed and granulated and calcined at 350°C for 2 hours to obtain a powdery catalyst precursor (spray Conditions: air inlet temperature 345°C, air outlet temperature 125°C, atomization disc rotation speed 220...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com