Reactive extrusion process for producing barium hydrogen phosphate
A reactive extrusion, barium hydrogen phosphate technology, applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., can solve the problems of large particle size of barium hydrogen phosphate, affecting product performance, mixing by-product impurities, etc., to achieve production. The effect of low cost, lower energy consumption and shorter reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] A method for producing barium phosphate with reactive extrusion process, comprising the following steps:
[0030] (1) After mixing 1970g of barium carbonate and 2400g of sodium dihydrogen phosphate, the mixture is reacted and extruded on a twin-screw extruder, and a white muddy product is obtained through reaction and extrusion; the reaction extrusion temperature is 50°C;
[0031] (2) Store the product of step (1) in a sealed environment at 50°C, and ripen for 24 hours;
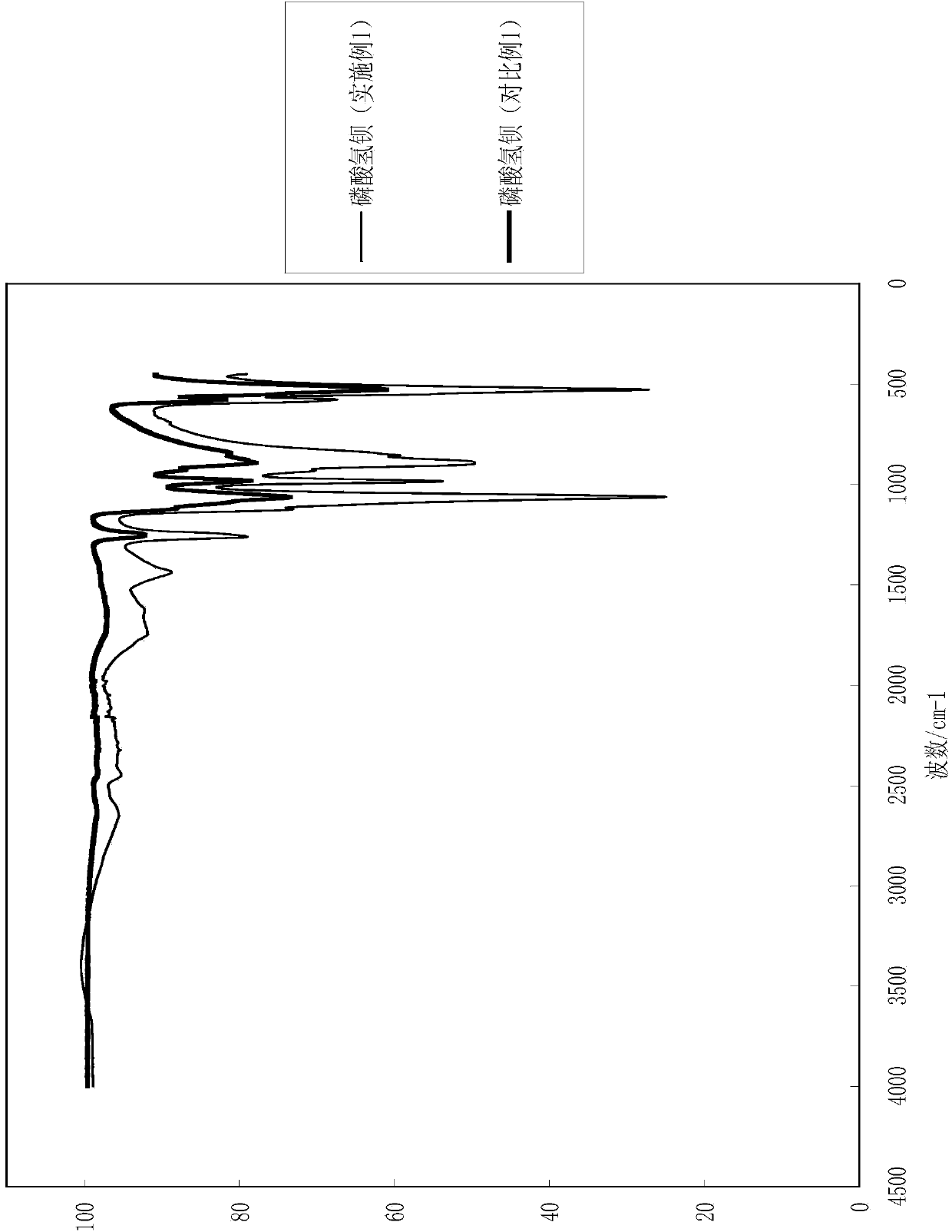
[0032] (3) Washing, filtering and drying the product of step (2) to obtain white barium hydrogen phosphate powder. After testing, the yield of the product obtained in this embodiment is 98%. The particle size analysis of the barium hydrogen phosphate of gained is shown in Table 1, and the infrared detection spectrum is shown in figure 1 .
Embodiment 2
[0041] A method for producing barium phosphate with reactive extrusion process, comprising the following steps:
[0042] (1) After mixing 2590g of barium bicarbonate and 2720g of potassium dihydrogen phosphate, the mixture is reacted and extruded on a twin-screw extruder, and a white muddy product is obtained through reaction extrusion; the reaction extrusion temperature is 40°C;
[0043] (2) Store the product of step (1) in a sealed environment at 20°C, and ripen for 48 hours;
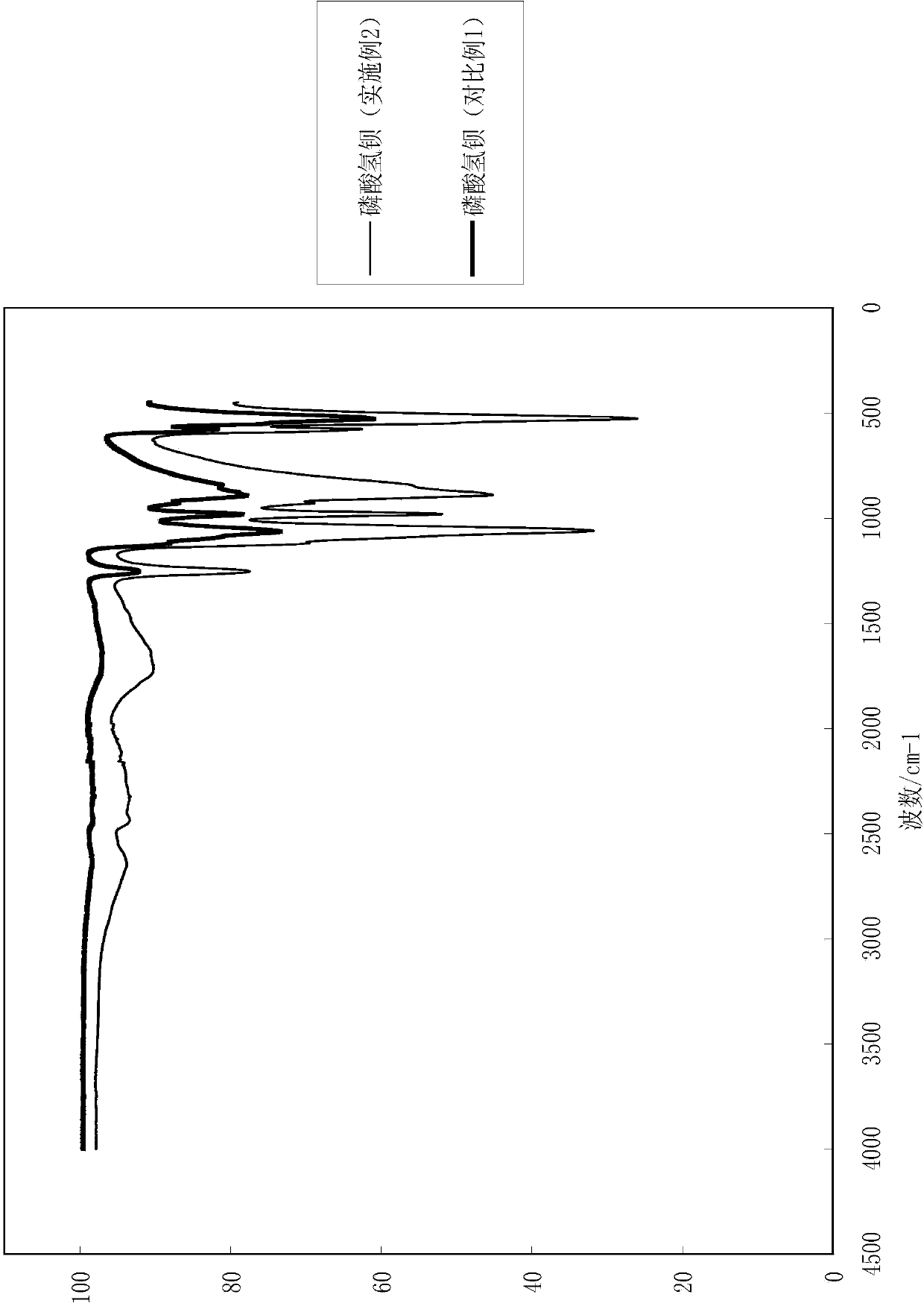
[0044] (3) Washing, filtering and drying the product of step (2) to obtain white barium hydrogen phosphate powder. After testing, the yield of the product obtained in this embodiment is 97%. The particle size analysis of the barium hydrogen phosphate obtained is shown in Table 2, and the infrared detection spectrum is shown in figure 2 .
[0045] The particle size analysis of the barium hydrogen phosphate that table 2 embodiment 2 and comparative example 1 make
[0046]
[0047] fig...
Embodiment 3
[0049] A method for producing barium phosphate with reactive extrusion process, comprising the following steps:
[0050] (1) After mixing 1710g barium hydroxide and 2300g ammonium dihydrogen phosphate, the mixture is reacted and extruded on a twin-screw extruder, and a white muddy product is obtained through reaction extrusion; the reaction extrusion temperature is 60°C;
[0051] (2) Store the product of step (1) in a sealed environment at 60°C, and ripen for 12 hours;
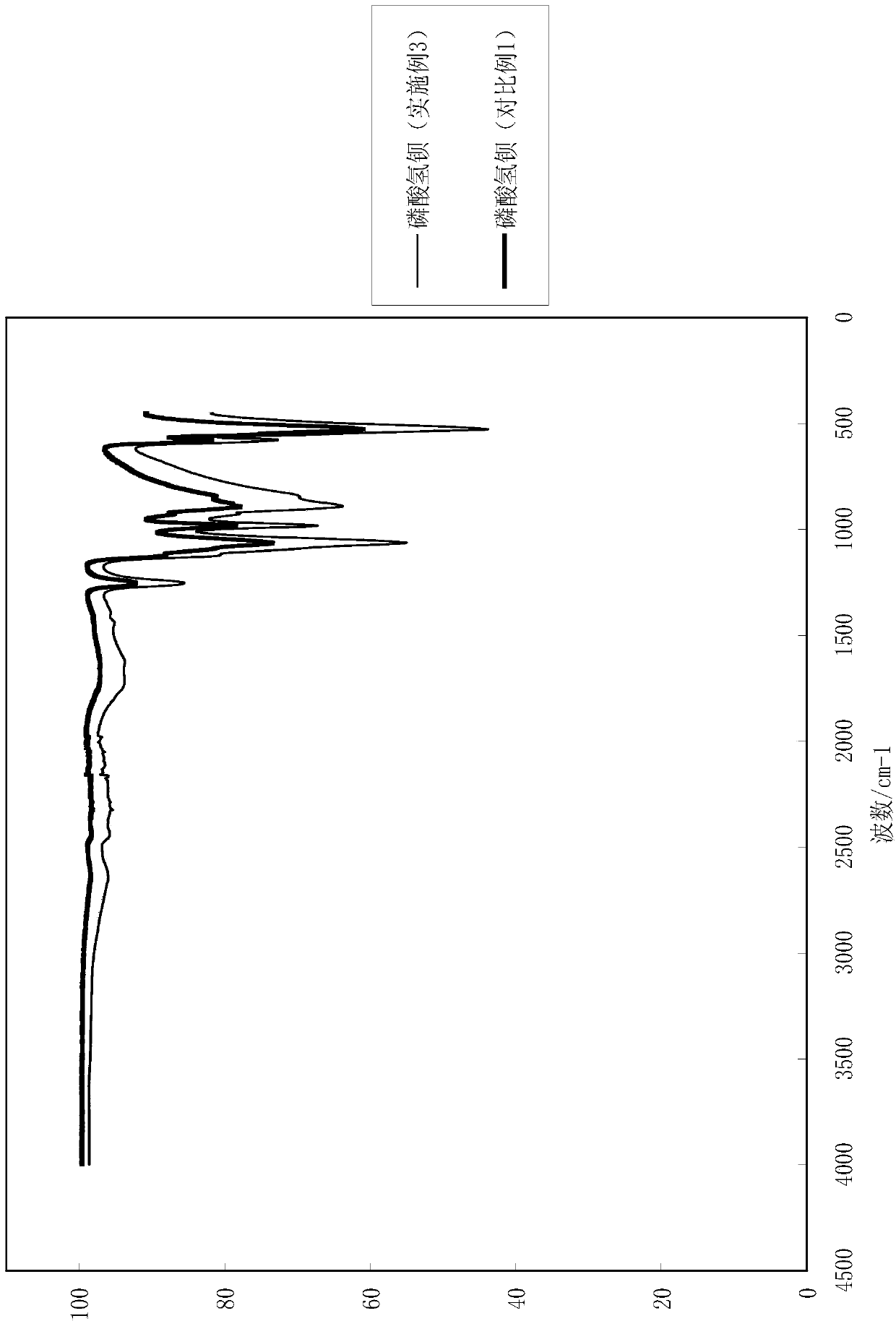
[0052] (3) Washing, filtering and drying the product of step (2) to obtain white barium hydrogen phosphate powder. After testing, the yield of the product obtained in this embodiment is 96%. The particle size analysis of the barium hydrogen phosphate obtained is shown in Table 3, and the infrared detection spectrum is shown in image 3 .
[0053] The particle size analysis of the barium hydrogen phosphate that table 3 embodiment 3 and comparative example 1 make
[0054]
[0055] image 3 It is...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com