Application of halogenated furanone compounds to preparation of HIV-1 (Human Immunodeficiency Virus-1)-resistant drugs
A HIV-1, halogenated furan technology, applied in the field of application of halogenated furan compounds in the preparation of anti-HIV-1 virus drugs, can solve the problems of anti-virus experiments of compounds that have not been reported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
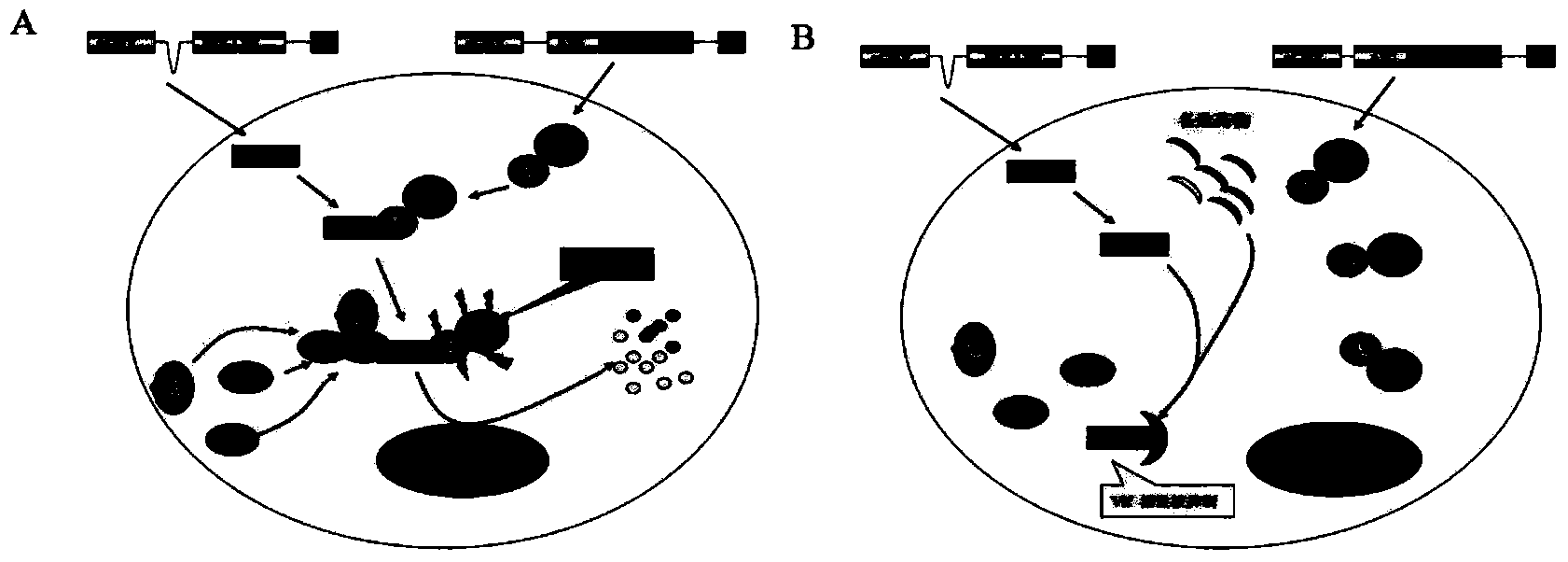
[0021] Vif is one of the essential proteins of HIV, and its main function is to antagonize the natural antiviral factor APOBEC3G in the host. APOBEC3G is a major threat to HIV-1 virus. It exists in HIV-1 natural host cells (such as CD4+ T cells and macrophages), can be packaged into HIV-1 virus particles, and is reverse transcribed in HIV-1 In the process, it exerts its strong antiviral effect. For this reason, HIV-1 itself encodes the Vif protein to specifically resist the antiviral activity of APOBEC3G, which can import APOBEC3G into the ubiquitin system and degrade it ( figure 1 A). Therefore, how to inactivate Vif is a very important target for the development of anti-HIV-1 virus drugs.
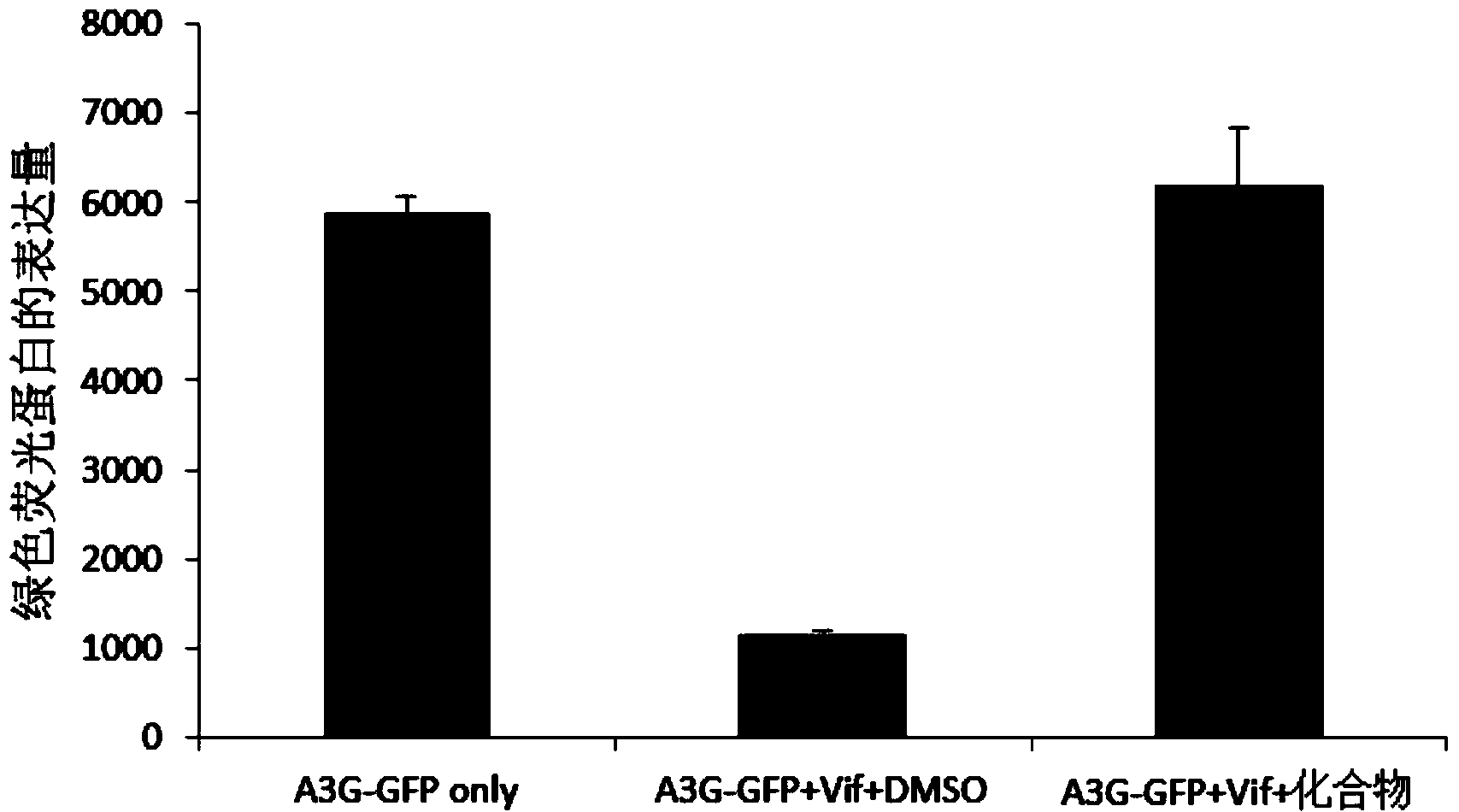
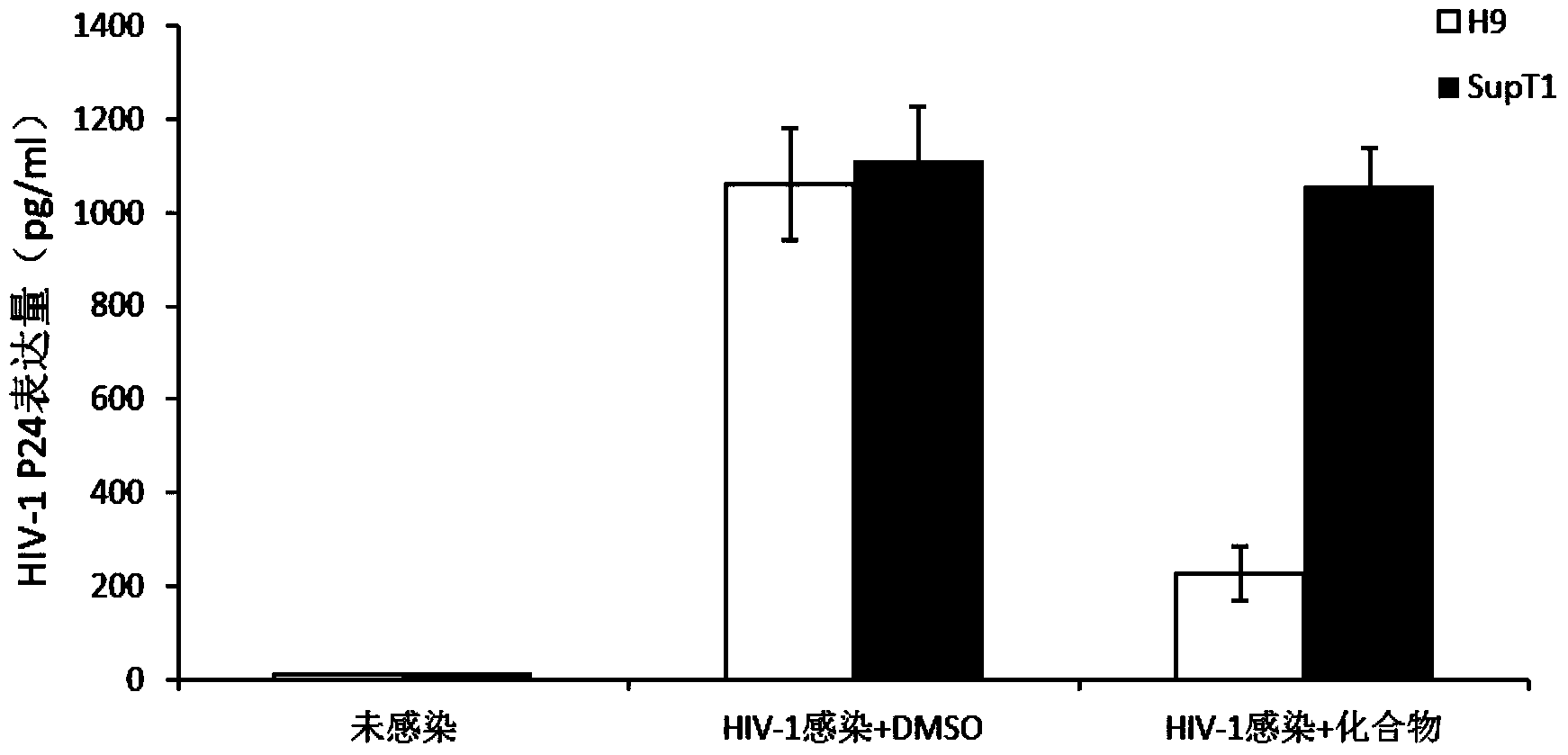
[0022] According to the molecular mechanism of Vif antagonizing APOBEC3G, several small molecule drugs that can prevent HIV Vif from degrading APOBEC3G were screened out. To this end, we will establish a simple living cell screening system, such as figure 1 As shown in B, plasmids ex...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com