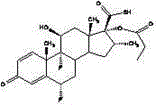
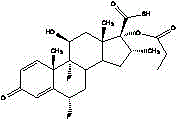
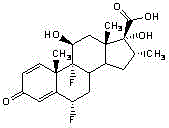
Highly pure fluticasone propionate preparation method
A kind of fluticasone propionate, high-purity technology, applied in the field of organic chemical synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0029] Add 5 g (12.61 mmol) of the first crude product solid oxidized with flumetasone into the flask, add 0.9 g of sodium hydroxide solution dissolved in 150 ml of water, stir at room temperature for 2 hours to dissolve, filter under reduced pressure, and use a small amount of Wash the filter cake and filter bottle with water, combine the filtrate and lotion, add 50ml of methanol to the flask, stir at room temperature for 20 minutes, add dropwise 2M hydrochloric acid to make the pH of the solution 2 to 4, stir for 2 hours, and filter under reduced pressure. The filter cake was washed with water until neutral, and dried at 70°C to obtain 4.9 g with an HPLC purity of 99.7%.
example 2
[0031] Put 5 grams (11.05 mmol) of the crude solid in the second step into the flask, add 0.8 grams of sodium hydroxide solution dissolved in 200 ml of water, stir at room temperature for 2 hours to dissolve, filter under reduced pressure, and wash the filter cake and Filter the flask, combine the filtrate and washing liquid, add 80ml of methanol to the flask, stir at room temperature for 20 minutes, add dropwise 2M hydrochloric acid to make the pH of the solution 2 to 4, stir for 2 hours, filter under reduced pressure, and wash the filter cake with water until Neutral, dried at 70°C to obtain 5.0 g of solid with HPLC purity of 99.6%.
example 3
[0033] Put 5 grams of the crude product in the third step into the reaction flask, add 150ml of ethyl acetate, stir at room temperature to dissolve, then extract with 5×50ml of 3% aqueous sodium hydroxide solution, then wash with 2×50ml of water, and wash with anhydrous sodium sulfate Dry, concentrate under reduced pressure to dryness, add water and stir at room temperature, disperse the solid, filter under reduced pressure, and dry at 75°C to obtain 4.8 g with an HPLC purity of 99.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com