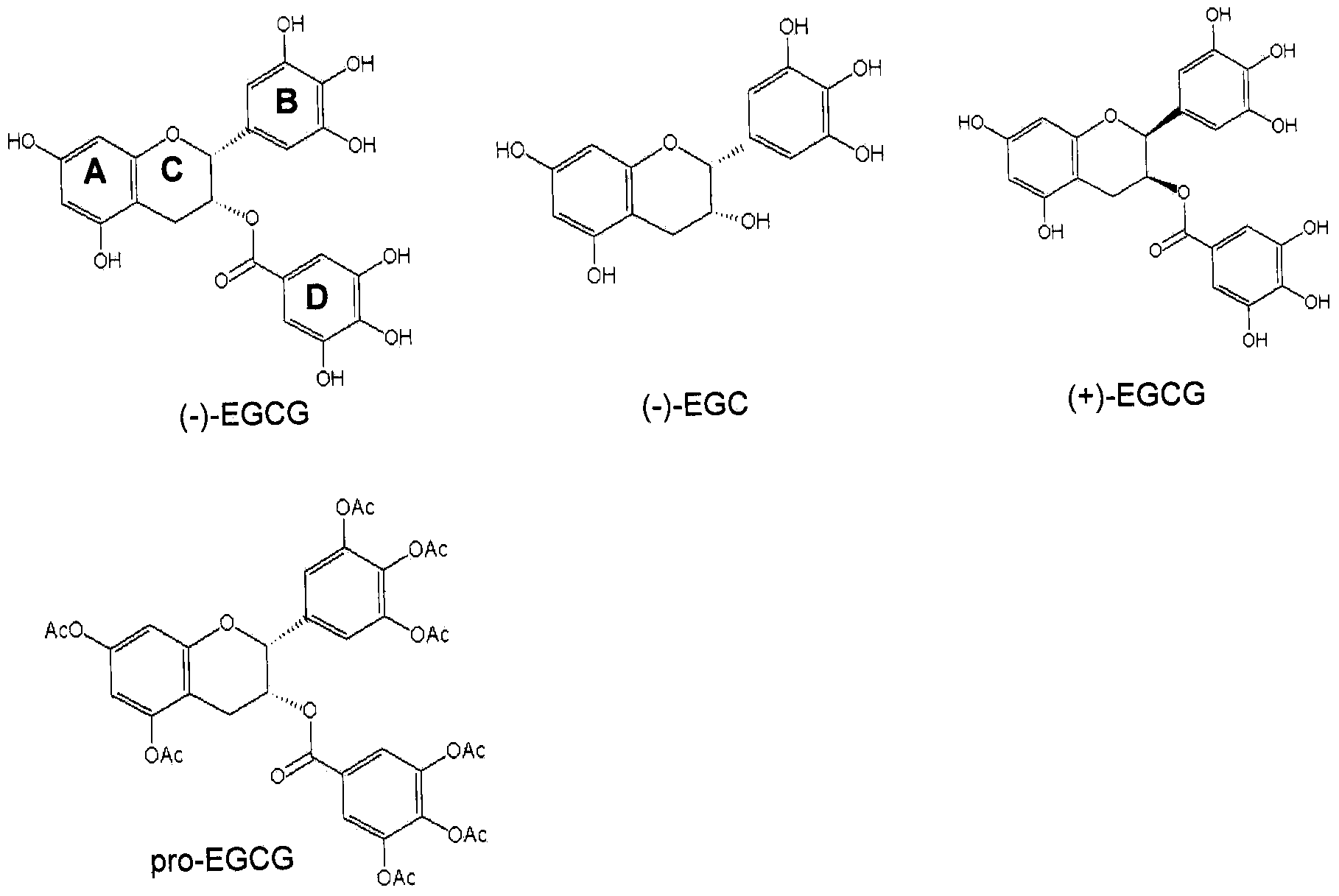
Synthetic epigallocatechin gallafe (EGGG) analogs
A technology of analogs and compounds, applied in the field of synthetic epigallocatechin gallate (EGCG) analogs, can solve the problems of reduced biological activity, short half-life, limited clinical use of green tea polyphenols, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0246] Preparation of cis-1,2,3,4-tetrahydronaphthalene-2,3-diol (12)
[0247] Acetone / H to 1,4-dihydronaphthalene (500mg, 3.84mmol) 2 Add NMO H to O (3.0 / 1.0mL) solution 2 O solution (1.43mL, 50%wt, 6.90mmol) and OsO 4 2-methyl-2-propanol solution (313 μL, 2.5% wt., 25 μmol). The mixture was stirred at room temperature for 16 hours. Add saturated Na 2 SO 3 aqueous solution (10 mL), and stirred for an additional 15 minutes. Join H 2 O (10 mL) and EtOAc (30 mL) and stirred for 5 minutes. The aqueous phase was extracted with EtOAc (4 x 30 mL). The combined organic phases were washed with brine (20 mL), and washed with anhydrous Na 2 SO 4 dry. The solution was concentrated by rotary evaporator and dried in vacuo to give the crude product which was chromatographed on silica gel (Hexane / EtOAc / CH 2 Cl 2 =5 / 1 / 1) to afford 521.7 mg (83%) of the title compound as a white solid. 1 H NMR (acetone-d 6 ,300MHz)δ7.13(m,2H),7.09(m,2H),4.11(t,J=5.4,2H),3.01(m,4H),2.36(s,2H); 1...
Embodiment 2
[0378] Inhibition of the chymotrypsin-like activity of purified 20S proteasomes
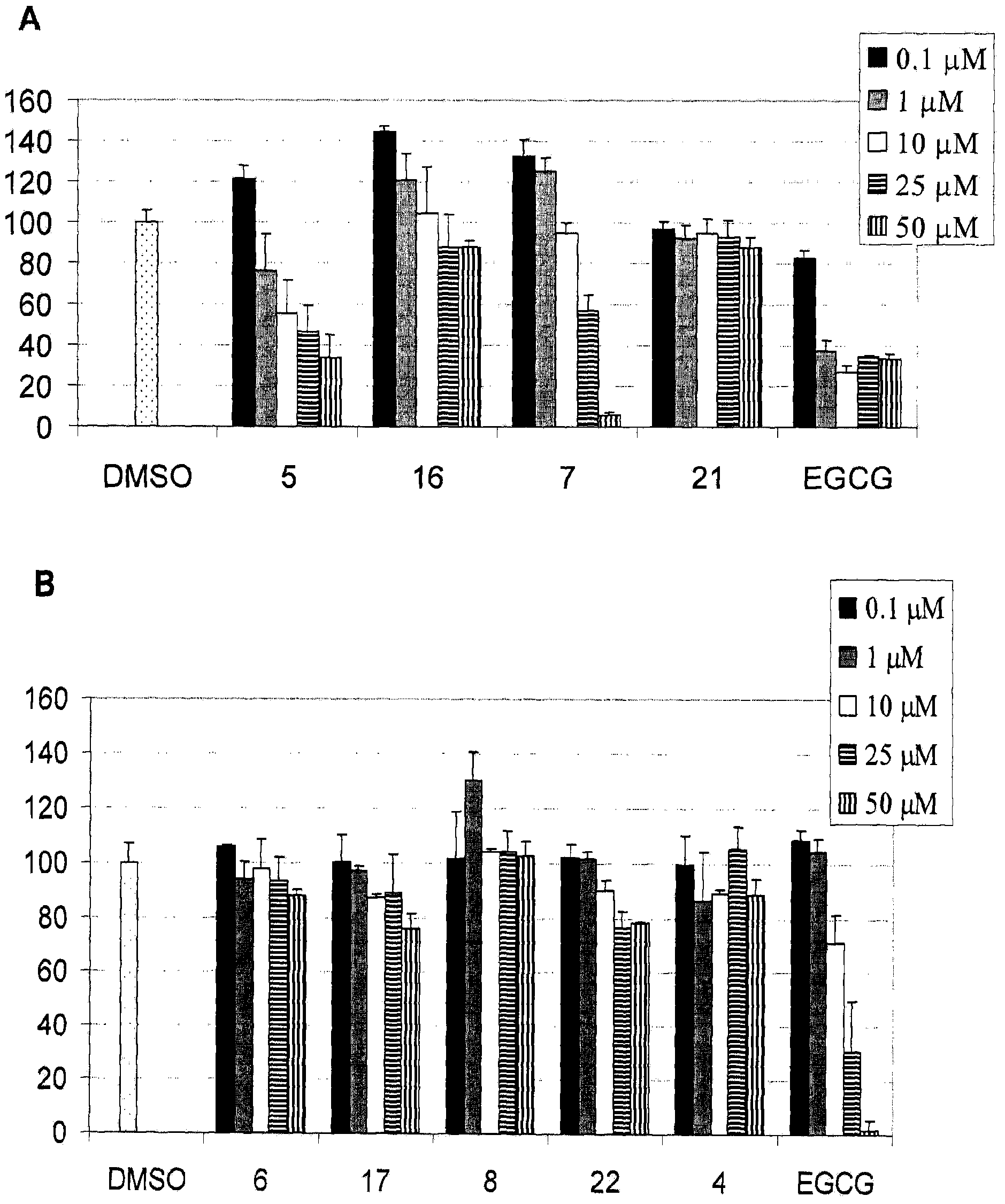
[0379] refer to figure 2 , EGCG effectively inhibited proteasomal chymotrypsin activity, which was consistent with our previous observations. Compound 5 is a substituted tetralin, which can be regarded as an analog of EGCG, which inhibits the chymotrypsin-like activity of the proteasome, IC 50 The value is 19 μM. Compound 16 lacking the gallate moiety did not inhibit proteasomal chymotrypsin activity even at a concentration of 50 μΜ. On the other hand, compound 7 (IC 50 =29 μM) was only slightly less active than EGCG or 5 despite the lack of gallate. Not surprisingly, compound 21 has no proteasome inhibitory activity even at 50 μM. When acetylated, the resulting derivatives 4, 6, 8, 17 and 22 showed no proteasome inhibition under these conditions.
Embodiment 3
[0381] COMT affects the proteasome inhibitory activity of derivatives 5 and 7
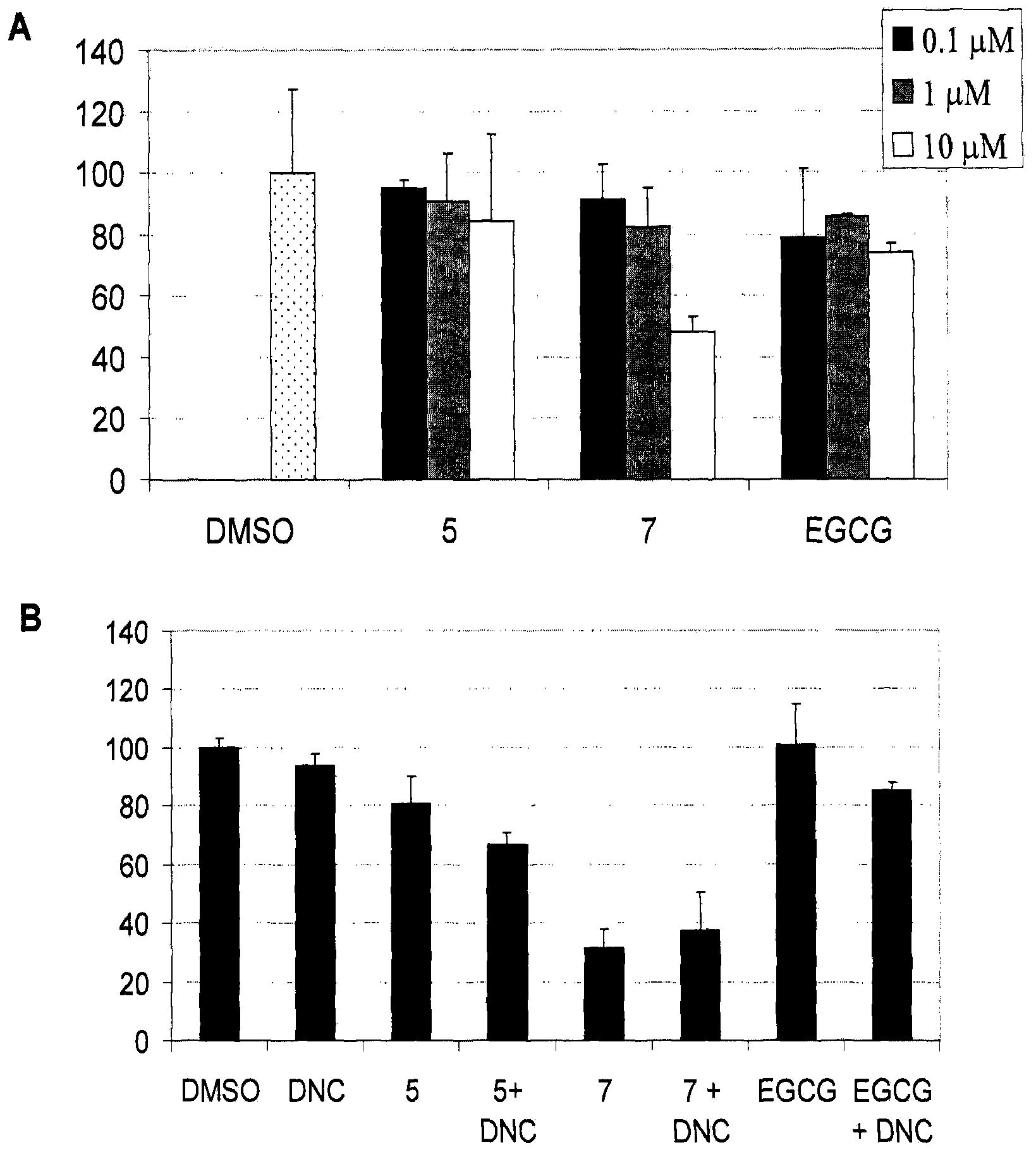
[0382] Lysates of human breast cancer MDA-MB-231 cells containing high COMT activity were treated with different concentrations of compound 5 or 7. image 3 It was shown that compound 7 at concentrations in the range of 1-10 μM inhibited between 18-51% of proteasome activity, while compound 5 only inhibited 10-16% of proteasome activity under the same conditions. Compound 7 was not expected to be more active than compound 5 in inhibiting the proteasomal activity of MDA-MB-231 cell lysates based on the data in Example 2. These results suggest that compound 5 may be more sensitive to biotransformation by COMT than compound 7. In contrast, 10 μM EGCG only inhibited the chymotrypsin-like activity of these cells by about 22%. Thus, consistent with previous reports, EGCG is also sensitive to methylation by COMT (H., Lu, X. Meng, C.S. Yang, Drug Metabolism and Disposition; 31; 572, 2003).
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com