Determination method for cadmium amount in copper slag
A technology of copper slag and constant volume, which is applied in the detection field, can solve problems such as unsuitable for popularization and application, many interfering elements, and high analysis cost, and achieve the effects of good precision, fast analysis speed, and accurate and reliable data
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
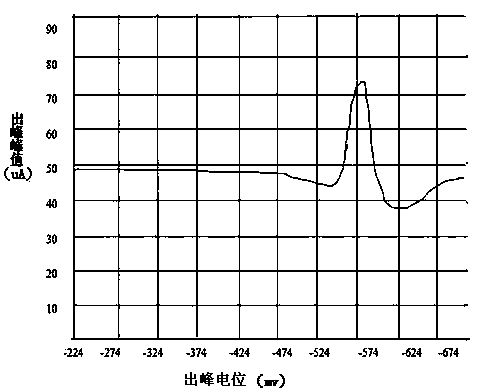
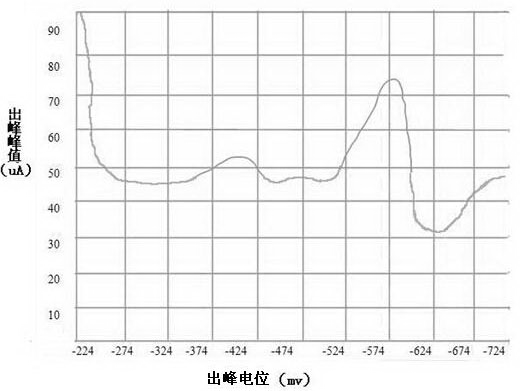
[0039]Weigh 5 parts of 0.1000g copper slag into a 100mL beaker, add 5, 8, 10, 12, 15mL of hydrochloric acid (ρ1.19g / mL) and heat for a few minutes, then add 3mL of nitric acid (ρ1.42g / mL) to test completely disassembled. Evaporate to a small volume, add 3-5mL hydrochloric acid (ρ1.19g / mL) and evaporate to near dryness. Rinse the watch glass and cup wall with water, and boil slightly to dissolve the soluble salt. Take it off, add ascorbic acid to make the yellow color of ferric iron in the solution completely disappear and the excess is about 0.1g. Transfer the solution into a 50mL volumetric flask filled with 25mL of 10% hydrochloric acid-10% ammonium acetate bottom solution, clean the beaker, dilute to the mark, shake well, and set aside. Pour the sample solution prepared above into the electrolytic cup, and take pictures with the oscilloscope polarograph. Anodization, two-pole derivative wave, origin potential -224mV, peak potential -600mV or so, measure its peak value, p...
Embodiment 2
[0044] Weigh 5 parts of 0.1000g copper slag into a 100mL beaker, add 10mL hydrochloric acid (ρ1.19g / mL) and heat for a few minutes, then add 1, 2, 3, 4, 5mL nitric acid (ρ1.42g / mL) to test The sample was completely decomposed, evaporated to a small volume, added 3-5mL hydrochloric acid (ρ1.19g / mL) and evaporated to near dryness. Rinse the watch glass and cup wall with water, and boil slightly to dissolve the soluble salt. Take it off, add ascorbic acid to make the yellow color of ferric iron in the solution completely disappear and the excess is about 0.1g. Transfer the solution into a 50mL volumetric flask filled with 25mL of 10% hydrochloric acid-10% ammonium acetate base solution in advance, clean the beaker, dilute to the mark, shake well, and set aside. Pour the sample solution prepared above into the electrolytic cup, and take pictures with the oscilloscope polarograph. Anodization, two-pole derivative wave, origin potential -224mV, peak potential -600mV or so, measure...
Embodiment 3
[0049] Weigh 10 parts of 0.1000g copper slag into a 100mL beaker, add 10mL hydrochloric acid (ρ1.19g / mL) and heat for several minutes, then add 3mL nitric acid (ρ1.42g / mL) to completely decompose the sample, steam to a small volume , add 3-5mL hydrochloric acid (ρ1.19g / mL) and evaporate to near dryness. Rinse the watch glass and cup wall with water, and boil slightly to dissolve the soluble salt. Take it off, add ascorbic acid to make the yellow color of ferric iron in the solution completely disappear and the excess is about 0.1g. Transfer the solution into 25mL pre-filled with 10% ammonium acetate-5% hydrochloric acid, 10% ammonium acetate-9% hydrochloric acid, 10% ammonium acetate-10% hydrochloric acid, 10% ammonium acetate-12% hydrochloric acid, 10% acetic acid Ammonium—15% hydrochloric acid, 10% hydrochloric acid—5% ammonium acetate, 10% hydrochloric acid—9% ammonium acetate, 10% hydrochloric acid—10% ammonium acetate, 10% hydrochloric acid—15% ammonium acetate, 10% hydr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com