Method for reducing pH value of lithium ion electrode material
An electrode material, lithium-ion technology, applied in the direction of battery electrodes, circuits, electrical components, etc., can solve the problems of high-temperature gas production, difficulty in getting coating, and inability to completely solve LTOPH value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
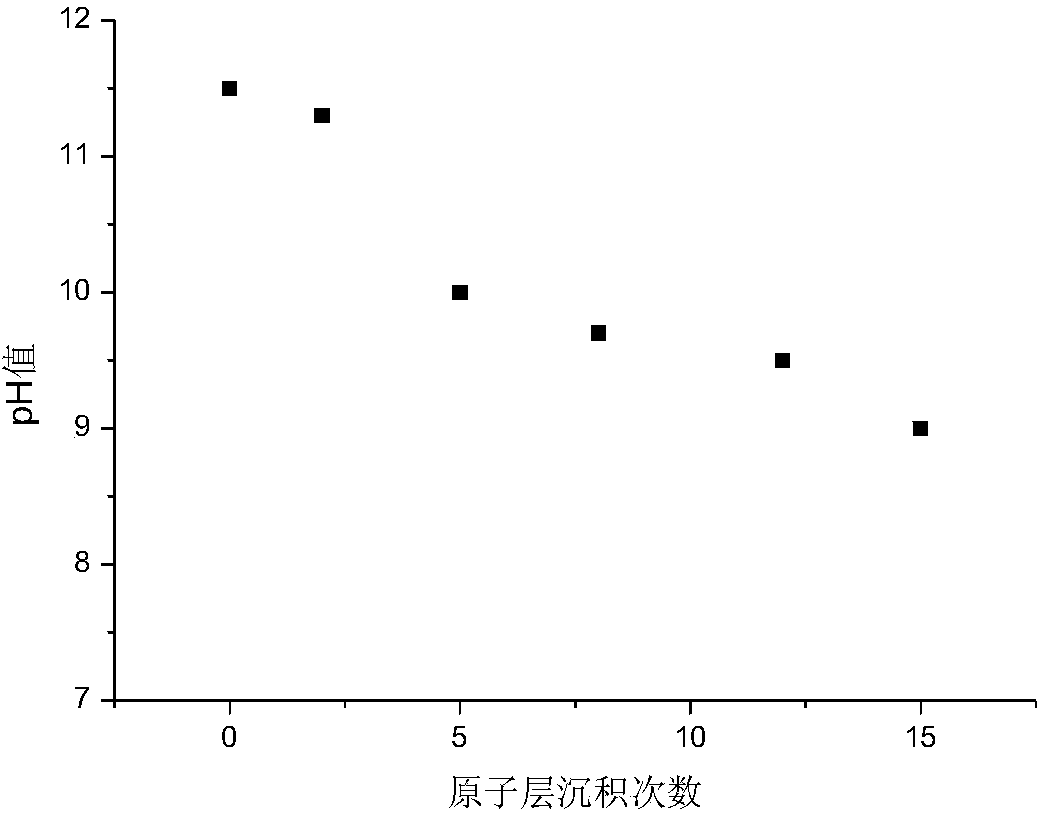
[0035] a. LiCoO 2 The electrode sample is placed in the reaction chamber of the atomic layer deposition instrument, vacuumed and the temperature of the reaction chamber is heated to 400 Kelvin, so that the electrode sample is kept at the set temperature for 25 minutes, and the air pressure in the reaction chamber is 10 millitorr;
[0036] b. Open the outlet valve, pulse the cleaning gas, and clean for 15 seconds;
[0037] c. Close the outlet valve, pulse gaseous trimethylaluminum for 0.01 seconds, and then keep for a period of 60 seconds;
[0038] d. Then open the gas outlet valve, pulse sweeping gas argon, and sweep for 1 minute; close the gas outlet valve, vacuumize, and remove excess reaction by-products;
[0039] e. Then close the outlet valve, pulse gaseous water for 10 seconds, and then keep it for 5 seconds;
[0040] f. Then open the outlet valve, pulse sweeping gas argon, and sweep for 1 minute; close the outlet valve again, vacuumize, and remove excess reaction by-p...
Embodiment 2
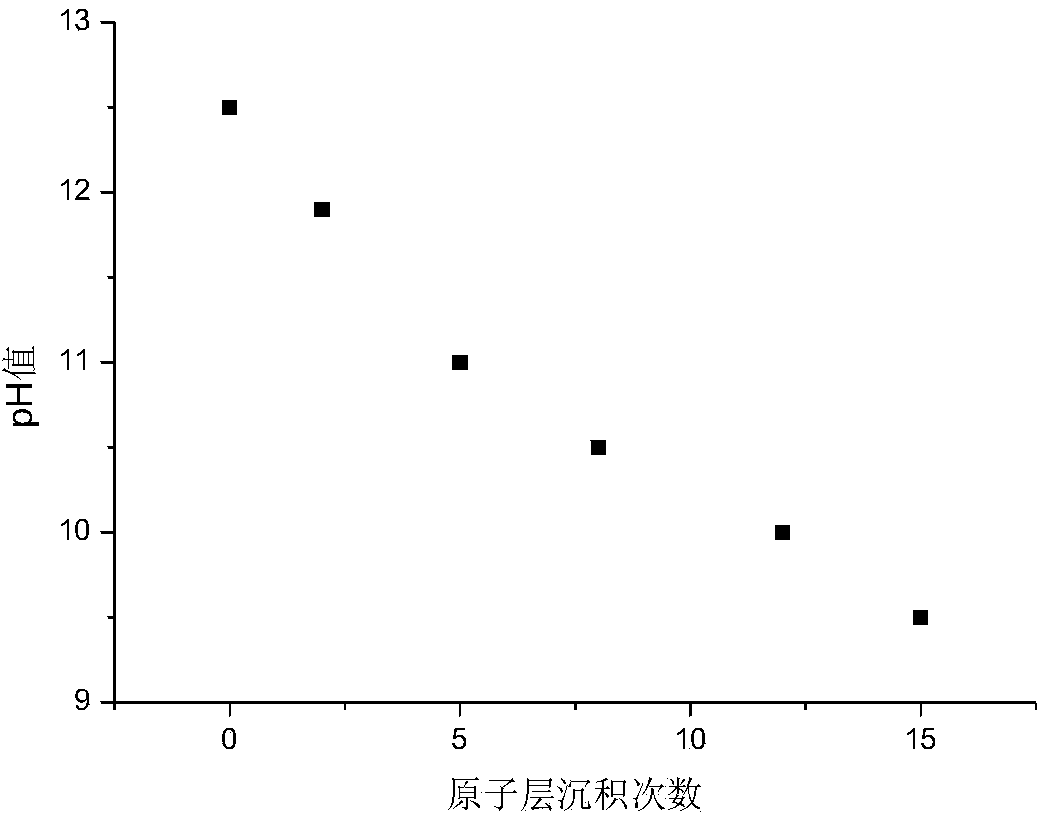
[0044] a. LiNi 0.8 mn 0.1 co 0.1 o 2 The electrode sample is placed in the reaction chamber of the atomic layer deposition instrument, vacuumed and the temperature of the reaction chamber is heated to 450 Kelvin, so that the electrode sample is kept at the set temperature for 5 minutes, and the air pressure in the reaction chamber is 10 millitorr;
[0045] b. Open the outlet valve, pulse sweeping gas nitrogen, and sweep for 3s;
[0046] c. Close the outlet valve, pulse gaseous aluminum trichloride for 10 seconds, and then keep it for 1 second;
[0047] d. Then open the air outlet valve, pulse purge nitrogen, and sweep for 0.1 minutes; close the air outlet valve, vacuumize, and remove excess reaction by-products;
[0048] g. Return to step c and execute step c and the following steps in a loop.
[0049] An electrode with a metal oxide thickness of approximately 2 angstroms was obtained. Such as figure 2 As shown, the electrode material treated by this method, especially...
Embodiment 3
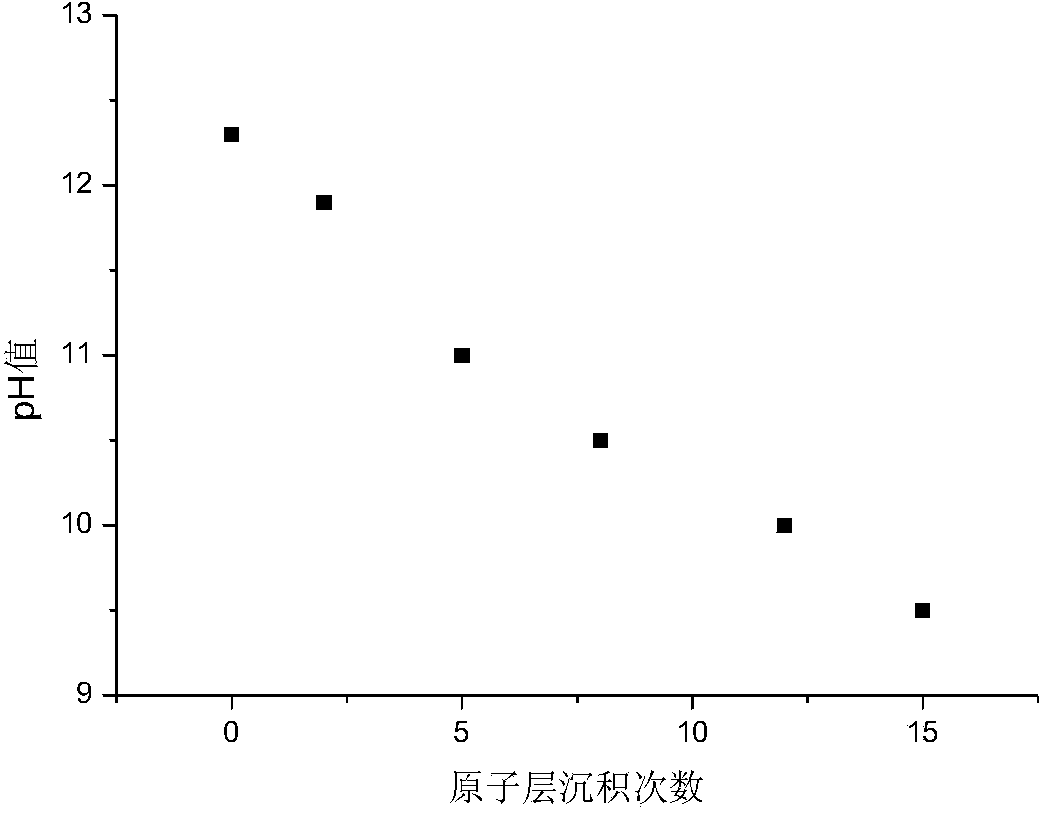
[0051] a. LiNi 0.6 mn 0.2 co 0.2 o 2 The electrode sample is placed in the reaction chamber of the atomic layer deposition instrument, vacuumized and the temperature of the reaction chamber is heated to 500 Kelvin, and the electrode sample is kept at the set temperature for 5 minutes, and the air pressure in the reaction chamber is 10 millitorr;
[0052] b. Open the outlet valve, pulse sweeping gas argon, and sweep for 10s;
[0053] c. Close the outlet valve, pulse gaseous tetraisopropyl titanate for 1 second, and then keep it for 20 seconds;
[0054] d. Then open the gas outlet valve, pulse sweeping gas argon, and sweep for 0.5 minutes; close the gas outlet valve, vacuumize, and remove excess reaction by-products;
[0055] e. Then close the outlet valve, pulse ozone for 1 second, and then keep it for 30s;
[0056] f. Then open the outlet valve, pulse sweeping gas argon, and sweep for 0.1 to 1 minute; close the outlet valve again, vacuumize, and remove excess reaction by-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com