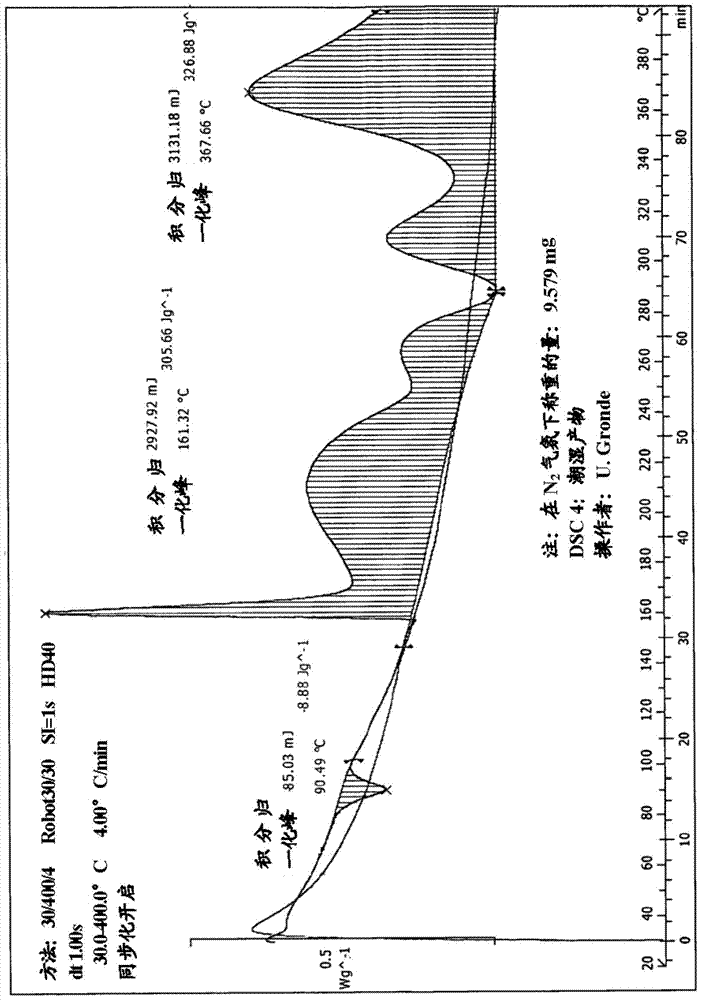
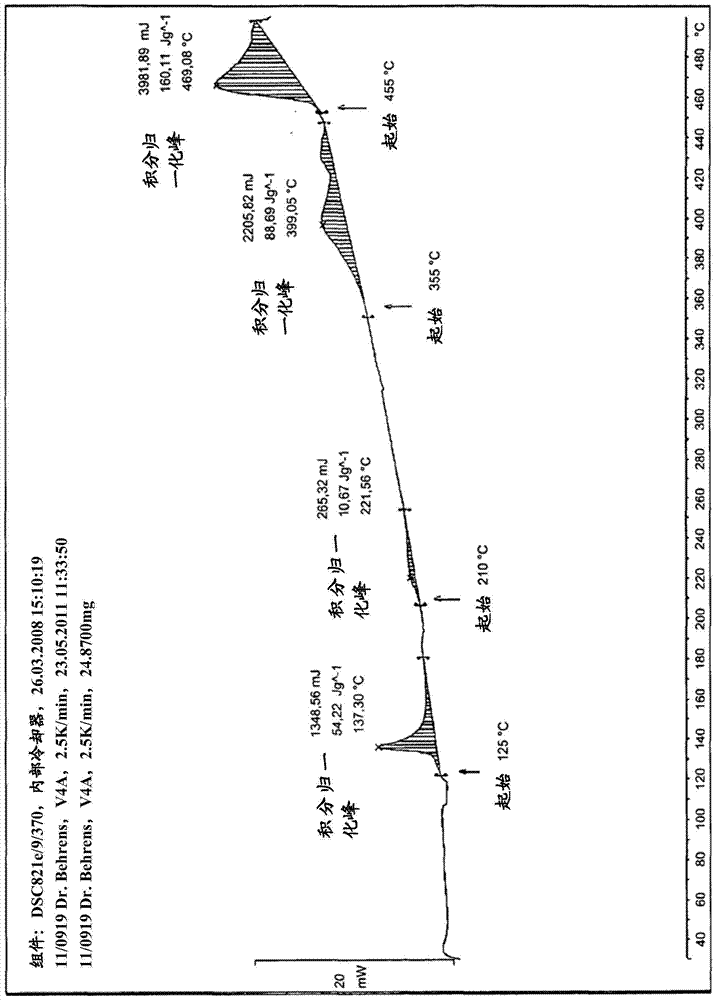
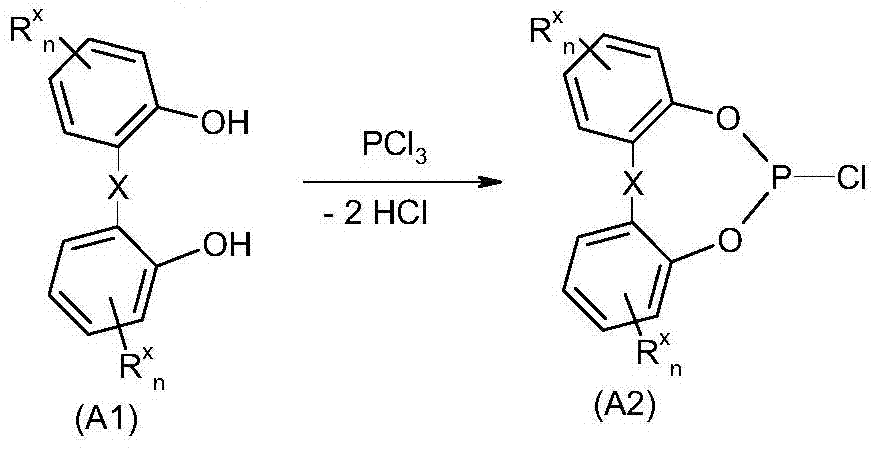
Method for purifying organic diphosphite compound
A technology of organic diphosphite and diphosphite, which is applied in the field of purifying organic diphosphite compounds, can solve the problems that the possible application of diphosphite is not described, and achieve the effect of simple and effective method and content reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0276] Example 1: Using methylimidazole Hydrochloride as a catalyst for the synthesis of 6-chlorodibenzo[d,f][1,2,3]dioxaphosphine
[0277] Combine 2,2'-dihydroxybiphenyl (931.1g, 5.0mol) and 1-methylimidazole under nitrogen The hydrochloride (0.9g, 7.6mmol) was placed in a 2000ml double-walled reactor and heated to an internal temperature of 142°C after the 2,2'-dihydroxybiphenyl was melted. Then start to introduce phosphorus trichloride (861.2 g, 6.26 mol) while stirring to ensure that the phosphorus trichloride does not fall on the wall of the hot reactor. The introduction rate is adjusted so that the connected HCl scrubber absorbs all the HCl formed. Adding the phosphorus trichloride requires a total of 3 hours. After adding the phosphorus trichloride, the mixture was stirred for another 3 hours at 140°C to obtain a yellow fluid reaction mixture. The reactor was then evacuated to a final vacuum of 16 mbar within 40 minutes to remove excess phosphorus trichloride. The fin...
Embodiment 2
[0278] Example 2: Synthesis of 6-chlorodibenzo[d,f][1,3,2]dioxaphosphine using N-methylpyrrolidone as a catalyst
[0279] Under nitrogen, 2,2'-dihydroxybiphenyl (88.0kg, 473mol) and N- were added to a 600-liter vessel provided with an inclined blade stirrer, condenser, exhaust gas discharge device through the scrubber, and a vacuum generating device. Methylpyrrolidone (0.337kg, 3.4mol). The mixture was melted by heating to an internal temperature of 140°C and at this time phosphorus trichloride (88.5 kg, 644 mol) was introduced at 140°C in a total of 7 hours. The reaction is slightly endothermic under vigorous release of HCl and gentle reflux. After all the phosphorus trichloride was added, the mixture was stirred at 140°C for another 9 hours, and then cooled to a container internal temperature of 50°C. Then the vessel was slowly evacuated at 50°C to a final pressure of 20 mbar (condenser temperature 5°C) to remove excess phosphorus trichloride. Excess phosphorus trichloride i...
Embodiment 3
[0280] Example 3: 6,6'-[[3,3',5,5'-tetrakis(1,1-dimethylethyl)-1,1'-biphenyl]-2,2'-diyl ]Bis(oxy)]bis-dibenzo[d,f][1,3,2]dioxaphosphine
[0281] Mix 3,3',5,5'-tetrakis (1,1-dimethylethyl)-1,1'-biphenyl-2,2'-diol while stirring at 85℃ within 60 minutes (92.7kg) the solution in the mixture of 1-methylimidazole (40.8kg) and toluene (313.5kg) was added 6-chlorodibenzo[d,f][1,3,2] obtained according to Example 2 Dioxaphosphaheptine (118.4 kg) in the melt. At the end of the addition there were two phases and these were stirred for another hour at 80°C. Then the internal temperature was raised to 90°C, the stirrer was turned off to allow the phases to separate and the phases were allowed to stand still for 20 minutes at 90°C to separate. 1-methylimidazole as the lower phase Hydrochloride (59kg) and this crystallized immediately. Of the upper phase remaining in the container 31 P-NMR confirmed that it is 6,6'-[[3,3',5,5'-tetra(1,1-dimethylethyl)-1,1'-biphenyl]-2,2'- Diyl]bis(oxy)]b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com