Application of cydiodine to treatment of keratoconjunctivitis
A technology of cydidinium iodine and eye drops, which is applied in the field of medicine and can solve the problems of eye irritation and inability to be widely used.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0059] Preparation example 1: the preparation of cediodine tablet
[0060] Formula (measured by the amount per tablet)
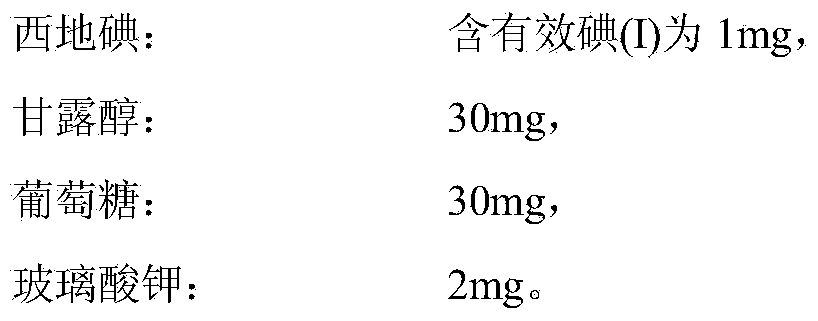
[0061] Cediiodine: Contains 1 mg of available iodine (I),
[0062] Glucose: 50mg,
[0063] Potassium Hyaluronate: 3mg.
[0064] Preparation method: fully mix cediodine and potassium hyaluronate, and then fully mix with glucose; wet granulate the mixed powder material with 50% ethanol, dry, and compress into tablets to make tablets containing 1 mg of available iodine (I) Agent, aluminum-plastic composite film sealed package, to get that, the sample is recorded as Ex1.
preparation example 2
[0068] Preparation example 2: the preparation of cediodine tablet
[0069] Formula (measured by the amount per tablet)
[0070] Cediiodine: Contains 1 mg of available iodine (I),
[0071] Mannitol: 50mg,
[0072] Potassium Alginate: 3mg.
[0073] Preparation method: refer to the method of Preparation Example 1, and this sample is marked as Ex2.
preparation example 3
[0076] Preparation example 3: the preparation of cediodine tablet
[0077] Formula (measured by the amount per tablet)
[0078] Cediiodine: Contains 1 mg of available iodine (I),
[0079] Glucose: 30mg,
[0080] Potassium Alginate: 5mg.
[0081]Preparation method: refer to the method of preparation example 1, and this sample is marked as Ex3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com