Rhizoma alismatis lipid-reducing concentrated pill
A technology of concentrated pills and Alisma, applied in the directions of pill delivery, plant raw materials, metabolic diseases, etc., can solve the problems of lack of efficient and safe clinical medication, increase of cholesterol concentration in bile, rhabdomyolysis, etc., to enhance the body's antioxidant capacity, The effect of improving dyslipidemia and reducing hepatic steatosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
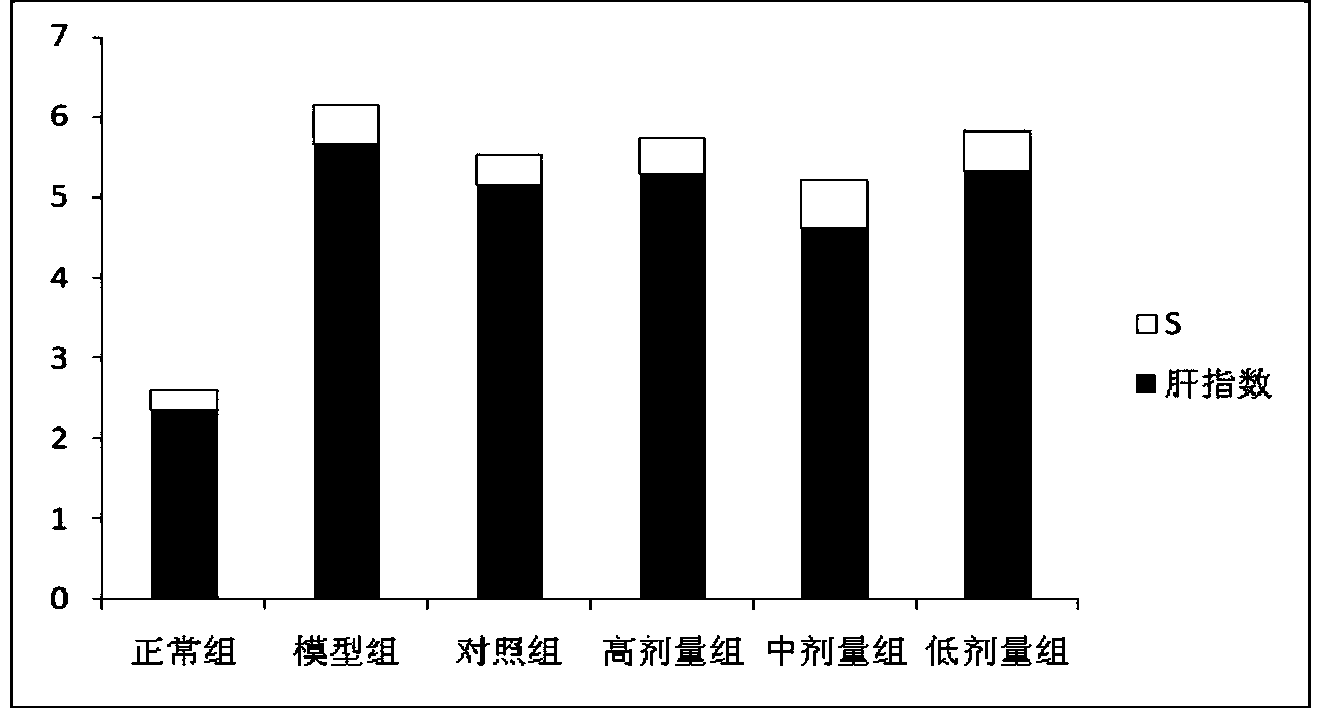
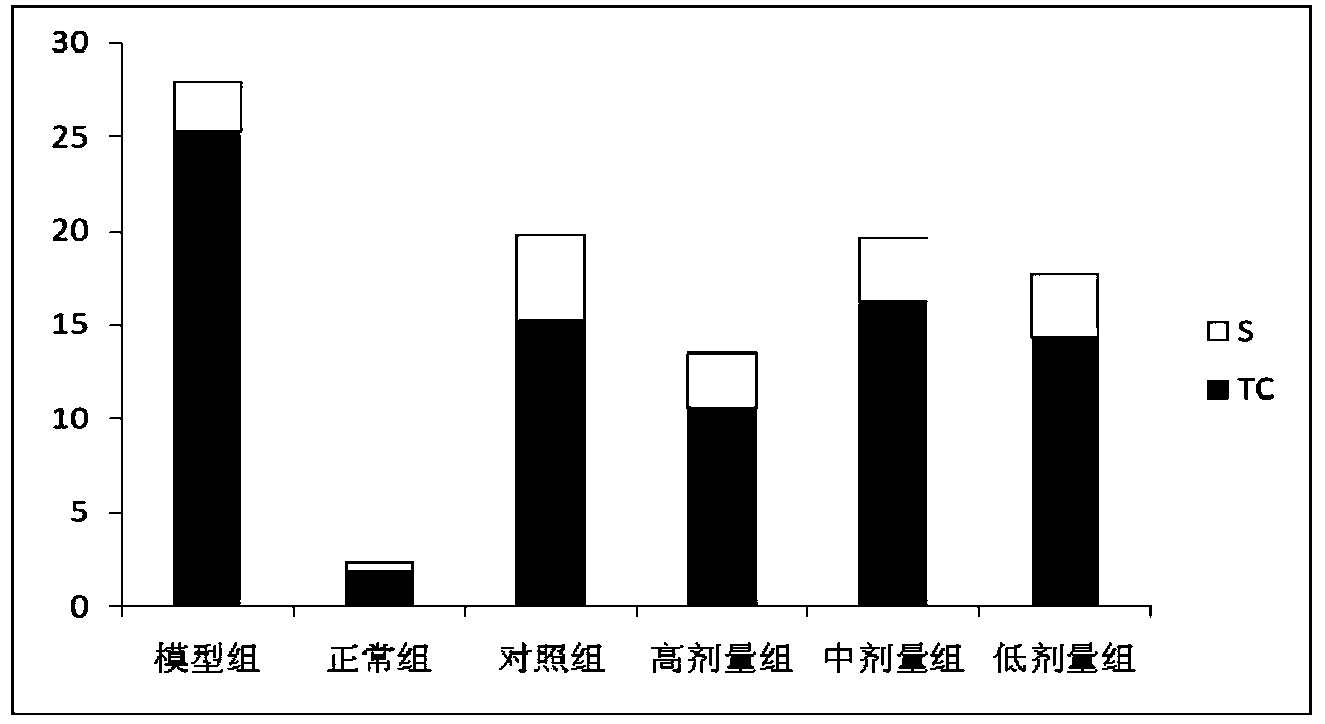
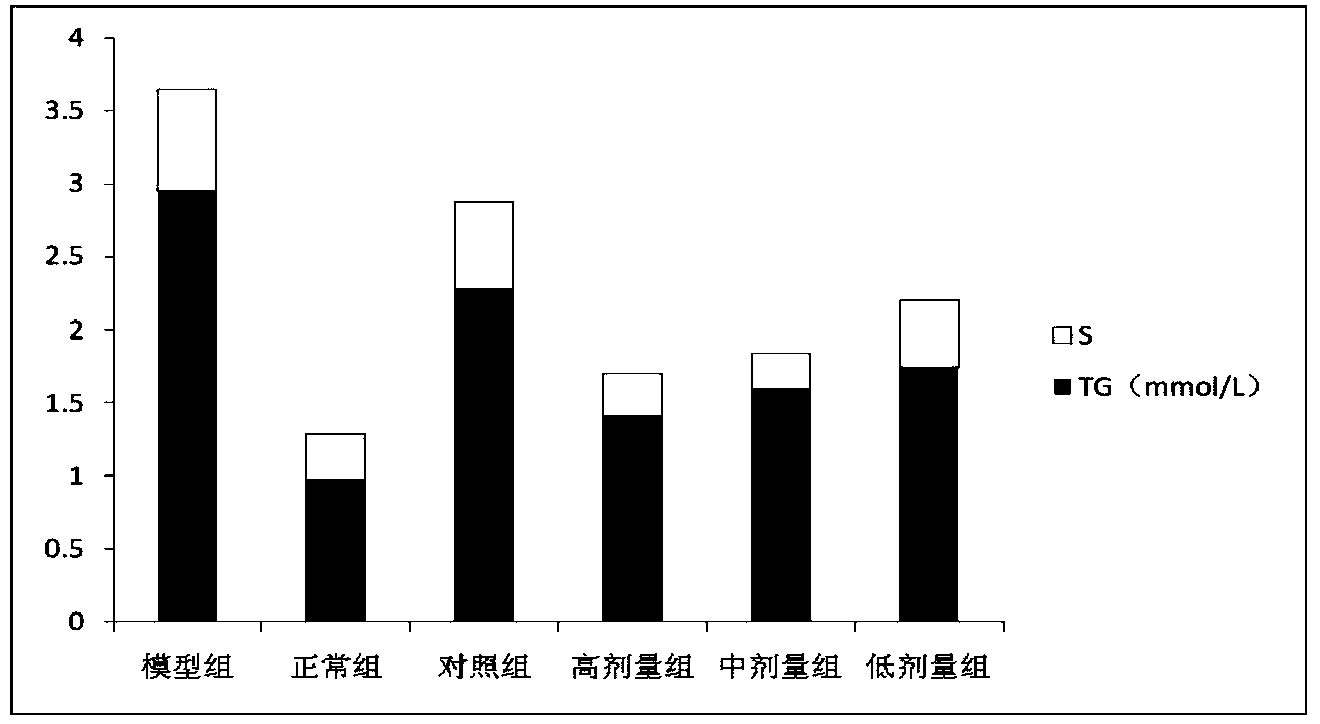
[0026] (1) Specific implementation method: a kind of Alisma reducing fat concentrated pill, which is mainly made of the following raw materials according to the following weight ratio: 20-30 parts of Alisma, 6-12 parts of emblica, fried Atractylodes macrocephala 6-12 parts, 6-12 parts of red yeast rice, 3-9 parts of lotus leaf, 3-9 parts of hawthorn.
[0027] A preferred weight ratio of the raw materials of the above-mentioned Chinese patent medicine is as follows: 25 parts of Alisma, 9 parts of Emlical, 9 parts of fried Atractylodes macrocephala, 9 parts of red yeast rice, 6 parts of lotus leaf, and 6 parts of hawthorn.
[0028] The Alisma alisma is preferably Alisma emblica; the emblica is preferably emblica produced in Fujian; and the red yeast rice is preferably Gutian red yeast rice.
[0029] Hyperlipidemia is mostly caused by dysfunction of the liver, spleen and kidney, phlegm turbidity and blood stasis. This prescription is aimed at the syndrome of stagnation of phlegm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com