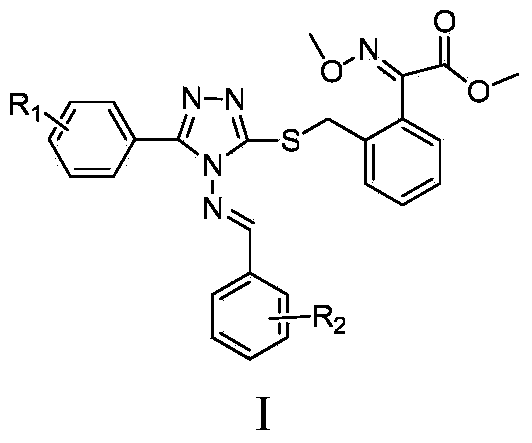
(E)-methyl 2-methoxyiminobenzeneacetate compound containing 1, 2, 4-triazole and preparation method and application thereof
A technology of methoxyimino and methyl phenylacetate, which is applied in the field of compounds and their preparation, and can solve problems such as low safety, environmental pollution, and monotony
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
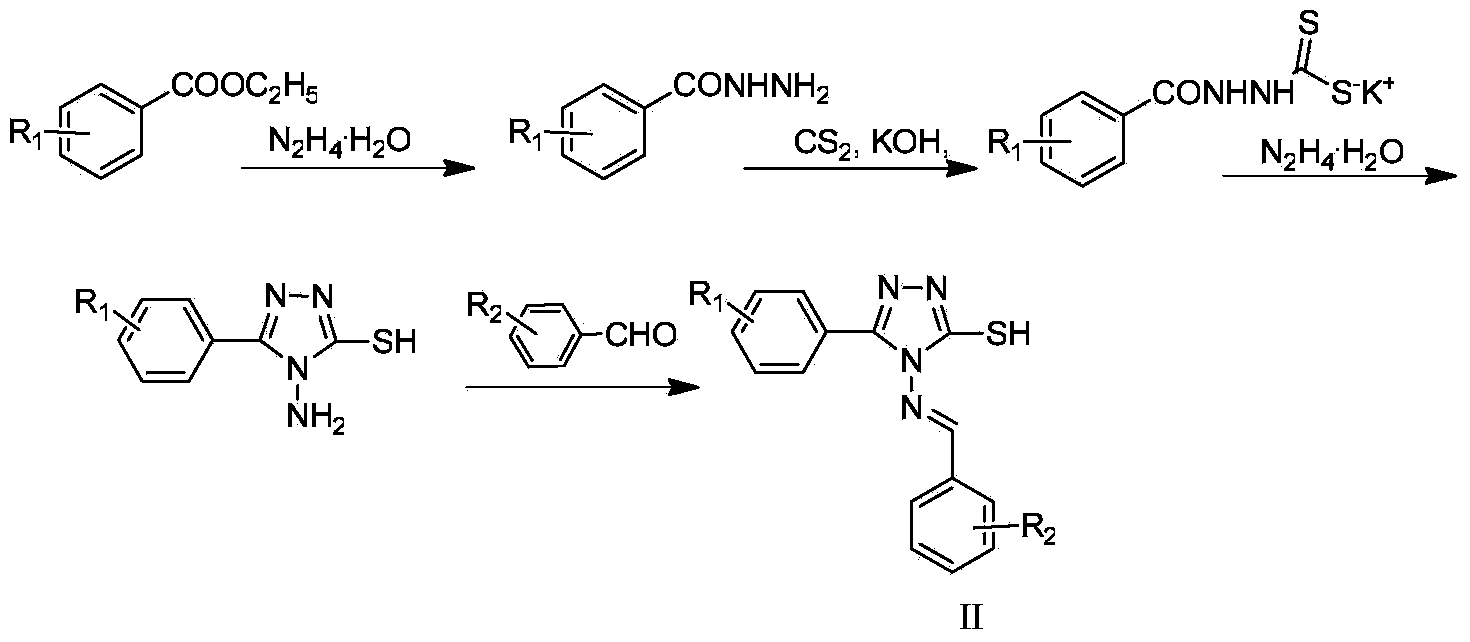
[0030] Synthesis of Example 1.3-aryl-4-amino-5-mercapto-1,2,4-s-triazole
[0031] (1) Synthesis of Benzohydrazide
[0032] Add 15g of ethyl benzoate to a 50mL round bottom flask, add ethanol, add 10mL of 85% hydrazine hydrate after stirring and dissolving the solid (when adding hydrazine hydrate at the beginning, the system often turns brownish yellow, gradually turns green, and with heating, finally become a colorless transparent liquid), heated to reflux for 4 hours, cooled and distilled off excess ethanol, a large amount of solids precipitated, filtered, and recrystallized with ethanol to obtain a white needle-like solid, which was dried to obtain 12.6 g of the product. Yield: 92.6%.
[0033] (2) Synthesis of benzoylhydrazine dithioformic acid potassium salt
[0034] Add 8g of benzohydrazide, 30mL of absolute ethanol, and 1:1.2 times KOH with benzohydrazide to a 100mL three-necked flask, stir and dissolve, then add 1:1 times of CS in an ice bath 2 , warming up to reflux ...
Embodiment 2
[0037] Example 2. Preparation of 3-mercapto-4-substituted phenylimino-5-phenyl-4H-1,2,4-s-triazole (II)
[0038] (1) Preparation of 3-mercapto-4-phenylimino-5-phenyl-4H-1,2,4-s-triazole (II-a)
[0039] In a 50mL single-necked round bottom flask, add 1.39g (10mmol) of 3-aryl-4-amino-5-mercapto-1,2,4-s-triazole, 1.06g (10mmol) of benzaldehyde, 30mL of absolute ethanol and Add 3 drops of glacial acetic acid, heat, reflux, and monitor by TLC. After the reaction was complete, cool to room temperature, filter with suction, and recrystallize with ethanol to obtain white rod-shaped crystals.
[0040] (2) Preparation of 3-mercapto-4-p-methoxyphenylimino-5-phenyl-4H-1,2,4-s-triazole (II-b) In a 50mL single-neck round bottom flask, Add 1.39 g (10 mmol) of 3-aryl-4-amino-5-mercapto-1,2,4-s-triazole, 1.36 g (10 mmol) of 4-methoxybenzaldehyde, 30 mL of absolute ethanol and 3 drops of ice Acetic acid, heating, reflux, TLC monitoring. After the reaction was complete, cool to room temperat...
Embodiment 3
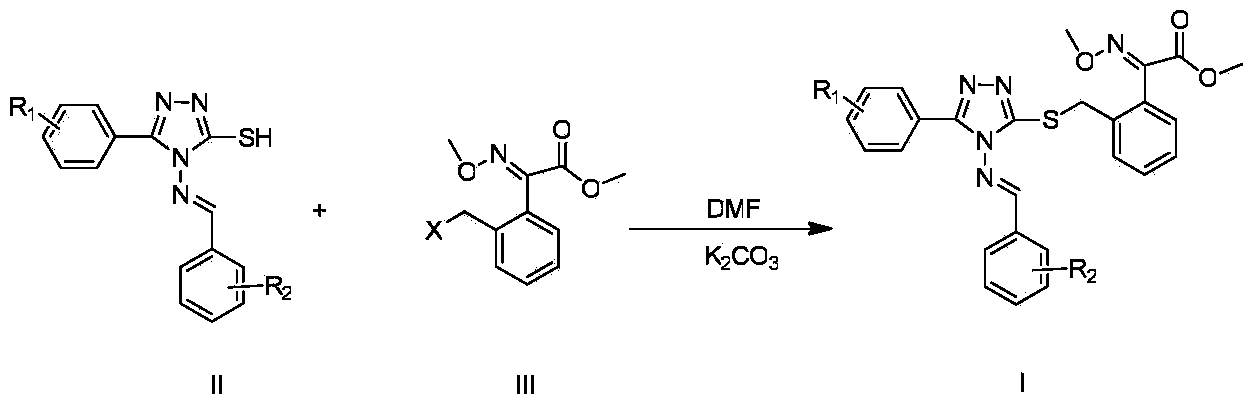
[0047]Example 3. Target compound (E)-2-(3-(4-(E)-2-substituted benzylideneamino-5-phenyl-4H-1,2,4-triazole-3-mercapto )-methylene)-phenyl)-3-methoxyiminoacetic acid methyl ester (Ia-Iv) preparation
[0048] (1) (E)-2-(3-(4-(E)-2-benzylideneamino-5-phenyl-4H-1,2,4-triazole-3-mercapto)-methylene Base)-phenyl)-3-methoxyiminoacetic acid methyl ester (I-a) preparation
[0049] Weigh 0.490g (1.75mmol) 3-mercapto-4-phenylimino-5-phenyl-4H-1,2,4-s-triazole 0.474g (1.75mmol) (E)-2-(2 -Bromomethyl)phenyl-3-methoxyiminoacetic acid methyl ester, 0.24g (1.75mmol) K 2 CO 3 , dissolved in 15ml DMF, and reacted at 80°C for 1h. Cool to room temperature, add 30mL of water, precipitate solid, filter with suction, recrystallize by heating with ethanol (a small amount), and filter with suction to obtain a white solid.
[0050] (1)(E)-2-(3-(4-(E)-2-p-methoxybenzylideneamino-5-phenyl-4H-1,2,4-triazole-3-mercapto )-methylene)-phenyl)-3-methoxyiminoacetic acid methyl ester (I-b) preparation
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com