Fluorescence in situ hybridization hTERT transfected external quality assessment cell line and preparation method thereof
A fluorescence in situ hybridization and cell line technology, applied in the field of fluorescence in situ hybridization hTERT transfection room quality assessment cell line and its preparation, can solve the problems of abnormal number of chromosomes and no quality control substances
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
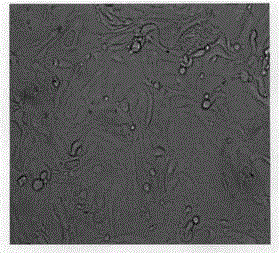
[0018] 1. Primordial cells: The primitive cells used are derived from trisomy 21, trisomy 18, trisomy 13, X or Y chromosome after diagnosis of chromosomal disease due to the needs of clinical diagnosis and treatment and have been diagnosed as one of the common abnormalities in chromosome number Abnormal remaining primary or passage living tissue cells that grow adherently, and the primary or passage living tissue cells may also involve living tissue cells with abnormal chromosome structure or No. 1 to No. Live tissue cells with abnormal number of chromosome 22. The cell types are fetal amniotic fluid, villous tissue and other cells.
[0019]2. Extraction of hTERT: ① Digestion of pClneo-hTERT: hTERT is located between the EcoRI and SalI sites of the plasmid pClneo-hTERT, and the multiple cloning site (MCS) of the pLXSNneo vector contains EcoRI and XhoI restriction sites. Take the pCIneo-hTERT plasmid and dissolve it in an appropriate amount of ultra-clean H 2 In O or TE buffe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com