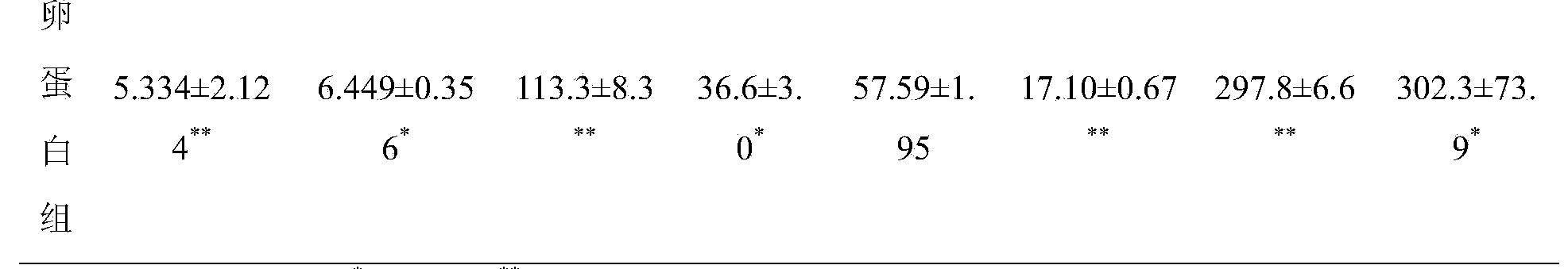Comprehensive evaluation method for passive cutaneous anaphylaxis test
A technology for skin allergies and reactions, applied in biological testing, material inspection products, testing pharmaceutical preparations, etc., can solve the problems of insufficient accuracy and high false negative rate, and achieve the effect of comprehensive evaluation indicators and avoiding false negatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] A kind of evaluation method for passive allergic reaction experiment, the steps are as follows:
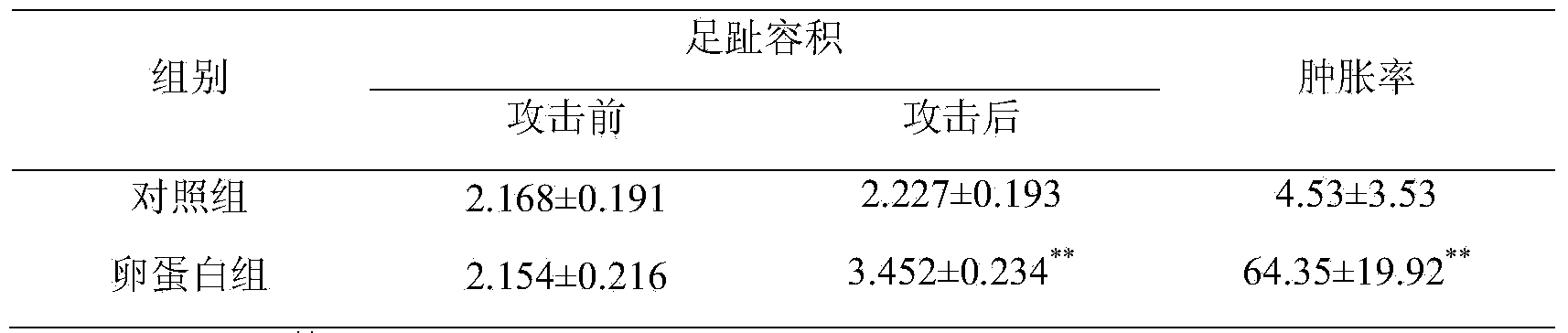
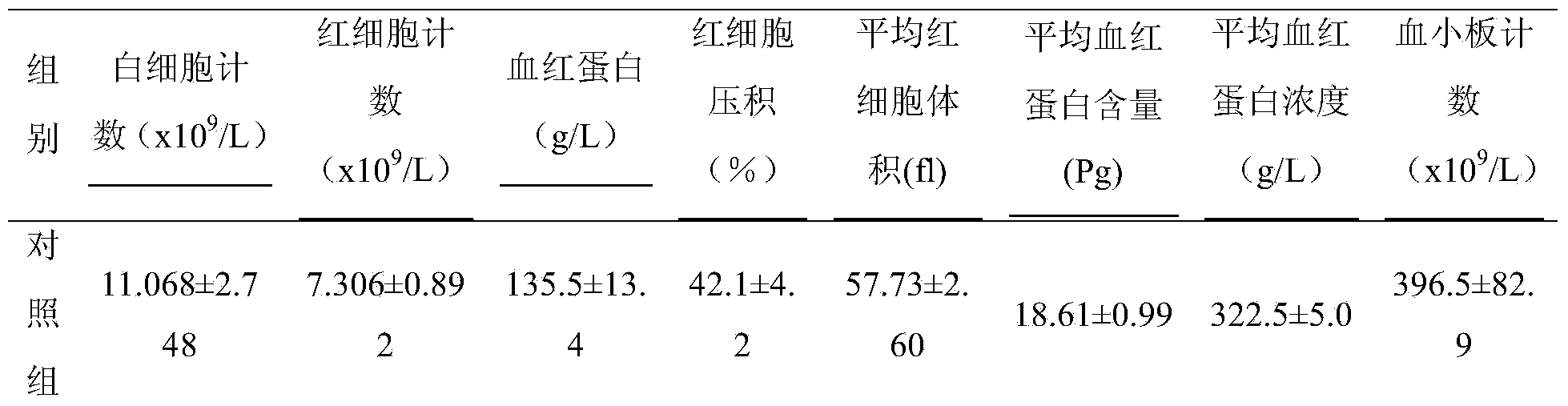
[0020] 1. Preparation of antiserum for passive skin allergic reaction test: Male BN rats were randomly divided into normal saline group and test drug group. Each group was sensitized on the 1st, 3rd, and 5th day, respectively, for a total of three times, and the animals in each group were subcutaneously given the clinically commonly used dose of the corresponding drug each time. Each group was challenged on the 12th day after the last sensitization, and the blood was collected before the challenge, and the supernatant was collected after centrifugation at 3000r / min for 15min, and stored at -20°C for future use.
[0021] 2. Passive skin allergy test: SD rats were randomly divided into normal saline group and test drug group, with 8 rats in each group. Rats were shaved at a place about 1.5cm away from the back midline on both sides of the back, with an area of about 4×4cm....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com