Drug for resisting HIV latency and applications thereof
A drug and application technology, applied in the field of drugs for the treatment of AIDS, can solve the problems of high toxicity and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] Example 1 Effect of Oxaliplatin on HIV Latent Induction and Activation
[0087] C11 cell is a latently infected cell model of HIV, which is obtained by infecting human T lymphocyte cell line Jurkat with HIV-1 lentivirus carrying EGFP reporter gene, through cell sorting and HIV integration detection, and has HIV integration but no expression EGFP T lymphocyte line. C11 cells are Jurkat stable strains that are infected by HIV lentivirus carrying the reporter gene GFP while not expressing, so they are used to screen for drugs that activate latent HIV-1 infection (see the technology disclosed in application number CN200810038851.X), and the preservation number is CCTCC NO .C200821.
[0088] In this example, 2×10 per well 4 For each cell, C11 cells were seeded in a 96-well plate, and 100 μl of 1640 medium (Gibco) containing 10% FBS (Gibco) was added to each well. After 24 hours, oxaliplatin (Oxaliplatin(1R,2R)-cyclohexane-1,2-diamine](ethanedioato-O,O')platinum(II), purch...
Embodiment 2
[0089] Example 2 Effect of Dilazole on HIV Latent Induction and Activation
[0090] Repeat Example 1, the only difference is that dilazep3-(4-{3-[(3,4,5-trimethoxyphenyl)carbonyloxy]propyl}-1,4-diazepan-1-yl)propyl-3 , 4,5-trimethoxybenzoate, purchased from Tocris) 10μM to treat the cells.
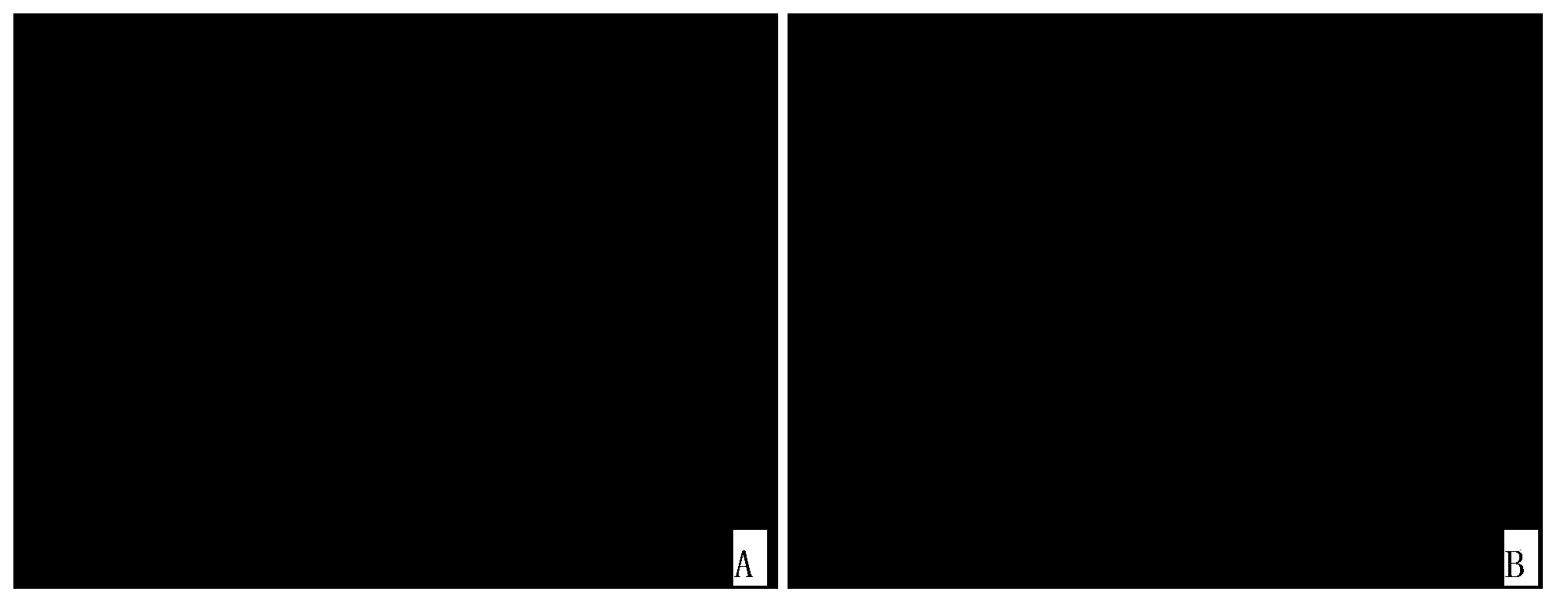
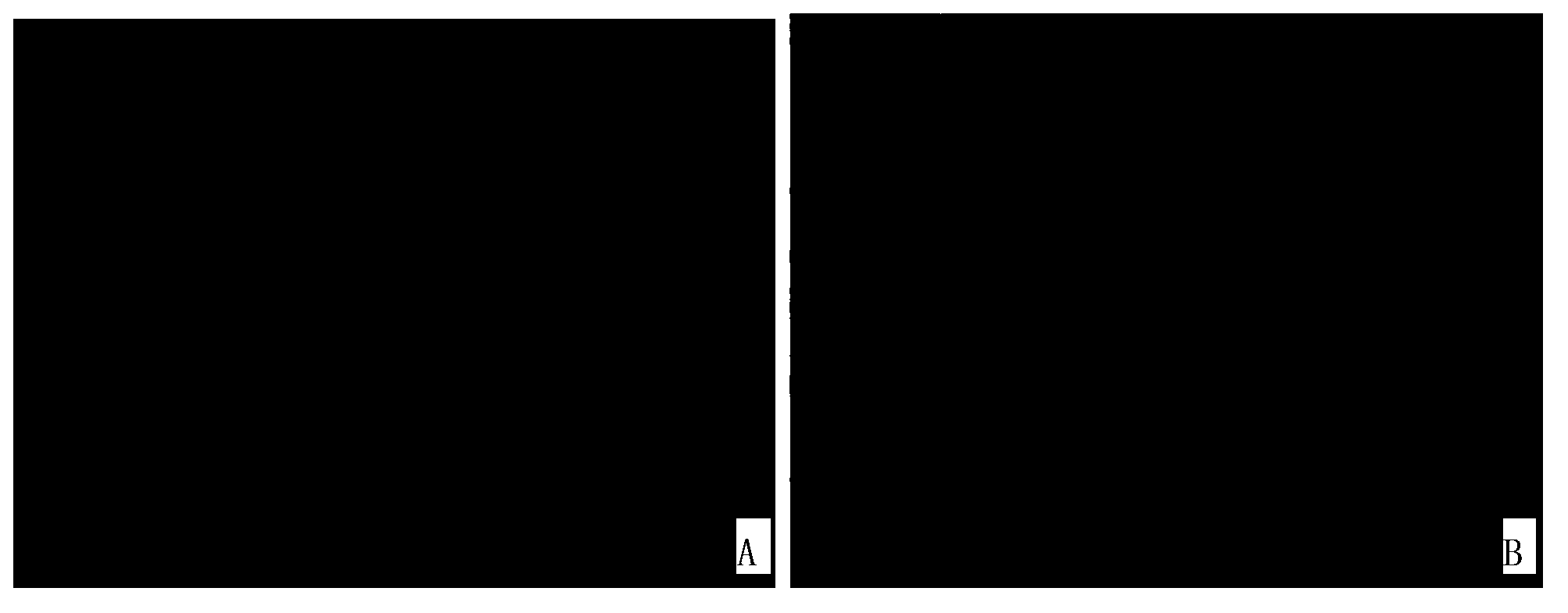
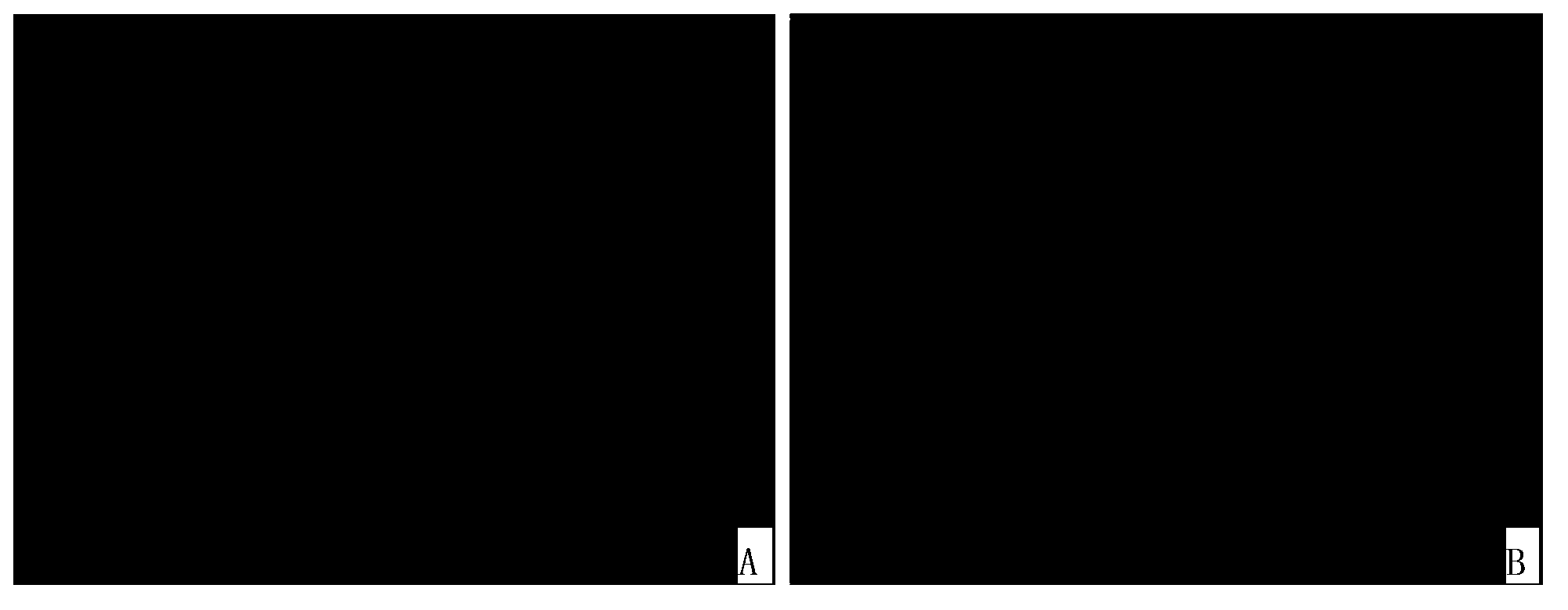
[0091] The result is as Figure 1~3 as shown, figure 1 The photos of the bright field and dark field of the undoped (mock) group; figure 2 It is the photo of the bright field and dark field of the oxaliplatin treatment group; image 3 Process brightfield and darkfield photographs for Dilazo. Fluorescent photographs have been destained to make the fluorescent cells white for easy identification on printed text.
[0092] The results showed that the proportion of green fluorescent positive cells in HIV latently infected cells was as high as 16% after treatment with oxaliplatin and dilazole respectively; the proportion of fluorescent positive cells in HIV latent infected cells not treate...
Embodiment 3
[0093] Example 3 Effects of different concentrations of oxaliplatin and dilazole on HIV latency-induced activation
[0094] Example 1 was repeated except that the cells were treated with oxaliplatin at final concentrations of 2.5 μM, 5 μM, 10 μM, and 20 μM and diazepam at concentrations of 10 μM, 20 μM, 40 μM, and 60 μM.
[0095] The result is as Figure 4 as shown, Figure 4 Shows the effect of different concentrations of oxaliplatin and dilazole on HIV latency-induced activation. C11 cells were treated with different concentrations of oxaliplatin and dilazole for 48 hours, and the proportion of GFP+ cells was analyzed by flow cytometry. The above data are the average value of the data obtained from 3 independent experiments. Sodium valproate (valproic acid, VPA; Sigma T8552) was used as a positive control drug, and its concentration was 0.5 μM, 1 μM, 2 μM, 4 μM.
[0096] Oxaliplatin (μM)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com