Meta-substituted tetrazole acetophenone as well as preparation method and usage thereof
A compound and general formula technology, applied in organic chemistry, drug combination, blood diseases, etc., can solve problems such as high bleeding risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] .
[0032] Reaction raw materials: self-made, conventional method.
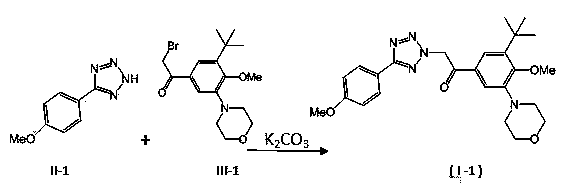
[0033] 1.60g (10 mmol) compound II-1 , 3.70 g (10 mmol) compound III-1 and 4.15 g (30 mmol) of solid potassium carbonate were stirred overnight in 20 mL of acetonitrile, and then heated to reflux for 3 hours.
[0034] The reaction mixture was cooled slightly and poured into 200 mL ice water, stirred, adjusted to pH = 4 with concentrated hydrochloric acid, extracted with 50 mL × 3 dichloromethane, combined organic phases, washed with brine, dried over anhydrous sodium sulfate, and dried on a rotary evaporator The solvent was evaporated, and the obtained residue was purified by column chromatography to obtain pure I-1 , yellow-white solid, MS, m / z = 472([M+Na] + ).
Embodiment 2-5
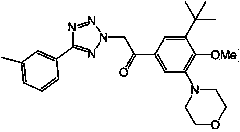
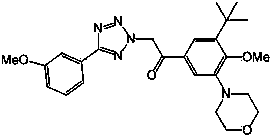
[0036] According to the method of Example 1, synthesized with general formula I of the following compounds:
[0037] 。
Embodiment 6
[0038] Example 6 In vitro platelet aggregation inhibition test
[0039] Pharmacological testing of substances in TRAP (thrombin receptor activating peptide)-induced platelet aggregation in 96-well plates. 3.13% sodium citrate solution was pre-added in the syringe, and then 20 mL of blood from healthy volunteers was drawn in, at 1500 g Platelet-rich plasma (PRP) was separated by centrifugation for 20 minutes and treated with 1 μL PGE1 solution (500 μg / mL in ethanol) / mL PRP. After incubation at room temperature for 5 minutes, they were centrifuged at 1200 g for 20 minutes to remove leukocytes. Transfer the leukocyte-free PRP to 15 mL PP tubes in batches at 5 mL / portion, and centrifuge at 3600 g to pellet the platelets. Then, decant the upper plasma layer and resuspend the platelet pellet from 5 mL of PRP in 1 mL of Tyrode (120 mM NaCl, 2.6 mM KCl, 12 mM NaHCO3, 0.39 mM NaH2PO4, 10 mM HEPES, 0.35% BSA, 5.5 mM Glucose, pH = 7.4), and Tyrode adjusted to a platelet count of 3 x...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com