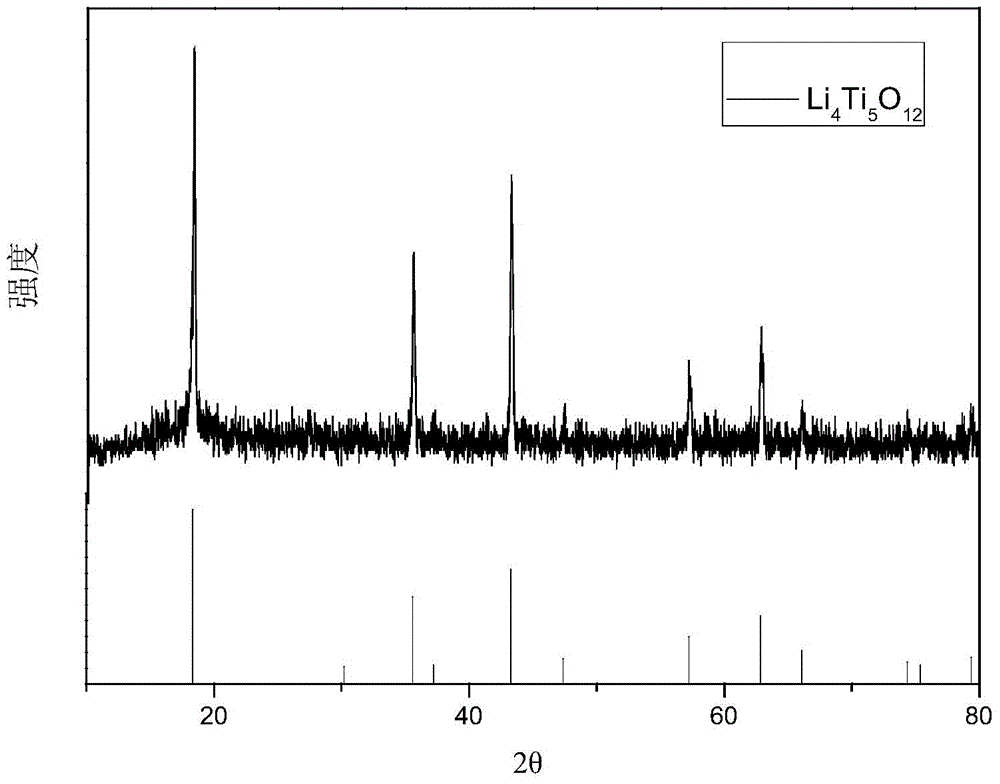
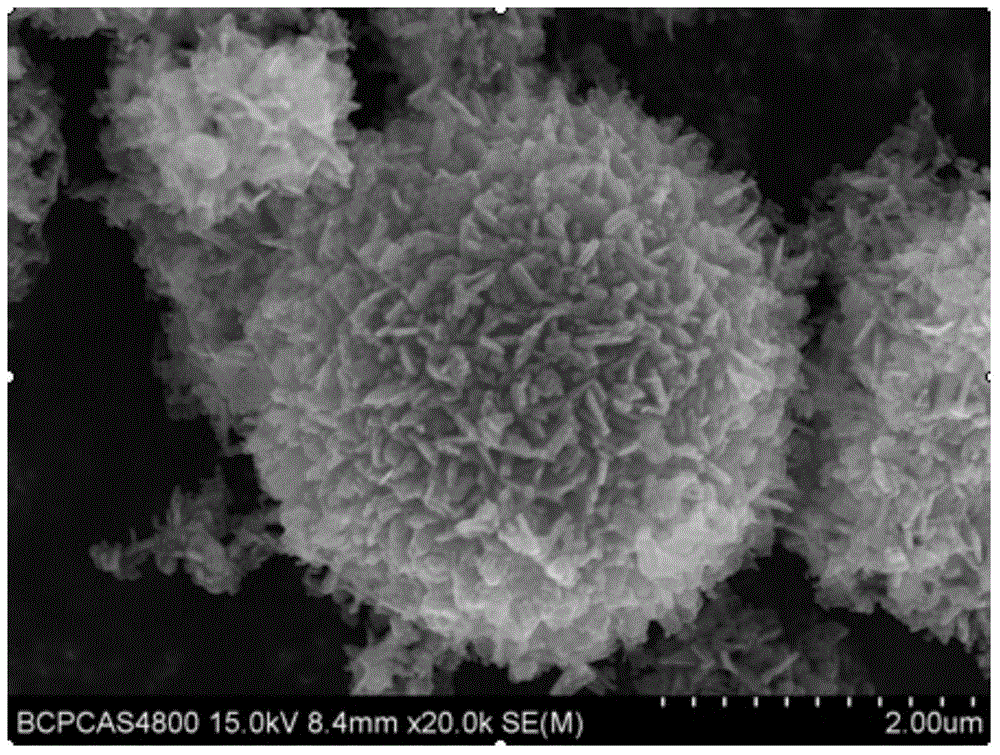
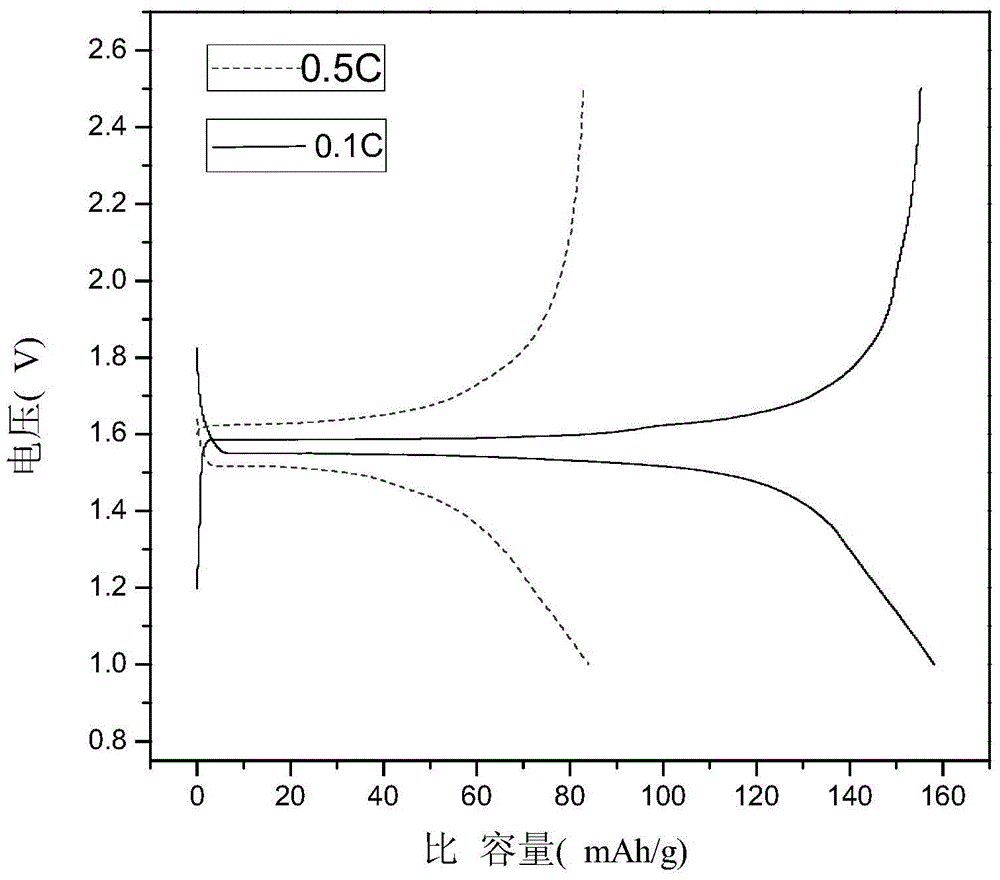
Hydrothermal synthesis preparation method for spherical Li4Ti5O12
A hydrothermal synthesis, spherical technology, applied in chemical instruments and methods, titanium compounds, inorganic chemistry, etc., can solve the problems of poor electronic conductivity, electrochemical performance, etc., and achieve easy operation, low raw material cost, and no pollution to the environment Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 1). Under the stirring action of a magnetic stirrer, add 1.324g HDA powder to 200ml absolute ethanol, add 0.8ml KCL solution after mixing, continue stirring for 10min, add 4.4ml TIP to the mixed solution. Continue to stir for 30 minutes and then stand for 18 hours.
[0023] 2). Centrifuge and wash the reaction product. Dry at 60°C for 12h to get pure TiO 2 .
[0024] 3). Put LiOH·H 2 O powder is added to the mixed solution of 60ml absolute ethanol and 20ml deionized water, and then the previously prepared TiO 2 0.69g, of which LiOH·H 2 O and TiO 2 The molar ratio of the powder is 4:5, and this process is always accompanied by a magnetic stirrer. After the reactants are added, continue to stir for 30 min.
[0025] 4). After the reactants are uniformly reacted, they are subjected to a hydrothermal reaction at 180°C for 12 hours.
[0026] 5). Centrifuge the reaction product, wash and filter, and dry at 60°C for 12 hours to obtain pure Li 4 Ti 5 O 12 powder.
Embodiment 2
[0028] 1). Under the stirring action of a magnetic stirrer, add 1.324g HDA powder to 200ml absolute ethanol, add 0.8ml KCL solution after mixing, continue stirring for 10min, add 4.4ml TIP to the mixed solution. Continue to stir for 30 minutes and then stand for 18 hours.
[0029] 2). Centrifuge and wash the reaction product. Dry at 60°C for 12h to get pure TiO 2 .
[0030] 3). Put LiOH·H 2 O powder is added to the mixed solution of 60ml absolute ethanol and 20ml deionized water, and then the previously prepared TiO 2 0.69g, of which LiOH·H 2 O and TiO 2 The molar ratio of the powder is 4:5, and this process is always accompanied by a magnetic stirrer. After the reactants are added, continue to stir for 30 min.
[0031] 4). After the reactants are uniformly reacted, they are subjected to a hydrothermal reaction at 180°C for 12 hours.
[0032] 5). Centrifuge the reaction product, wash, and dry at 60°C for 12 hours to obtain pure Li 4 Ti 5 O 12 powder.
Embodiment 3
[0034] 1). Under the stirring action of a magnetic stirrer, add 1.324g HDA to 200mL absolute ethanol, add 0.8ml KCL after mixing, stir for 10min, then add 4.4ml TIP to the mixed solution, and continue stirring after the reactants are added. Let stand for 18h after 50min.
[0035] 2). Centrifuge and wash the reaction product. Dry at 60°C for 12h to get pure TiO 2 powder.
[0036] 3). Put 0.432g LiOH·H 2 O was added to the mixed solution of 60ml absolute ethanol and 20ml deionized water, and then added the previously prepared TiO 2 0.69g, of which LiOH·H 2 O and TiO 2 The molar ratio of the powder is 4:5, and the process is always stirred with a magnetic stirrer. After the addition of the reactants is completed, stirring is continued for 30 minutes.
[0037] 4). After the reactants are uniformly reacted, they are subjected to a hydrothermal reaction at 180°C for 12h.
[0038] 5). Centrifuge the reaction product, wash, and dry at 60°C for 12 hours to obtain pure Li 4 Ti 5 O 12 powder. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com