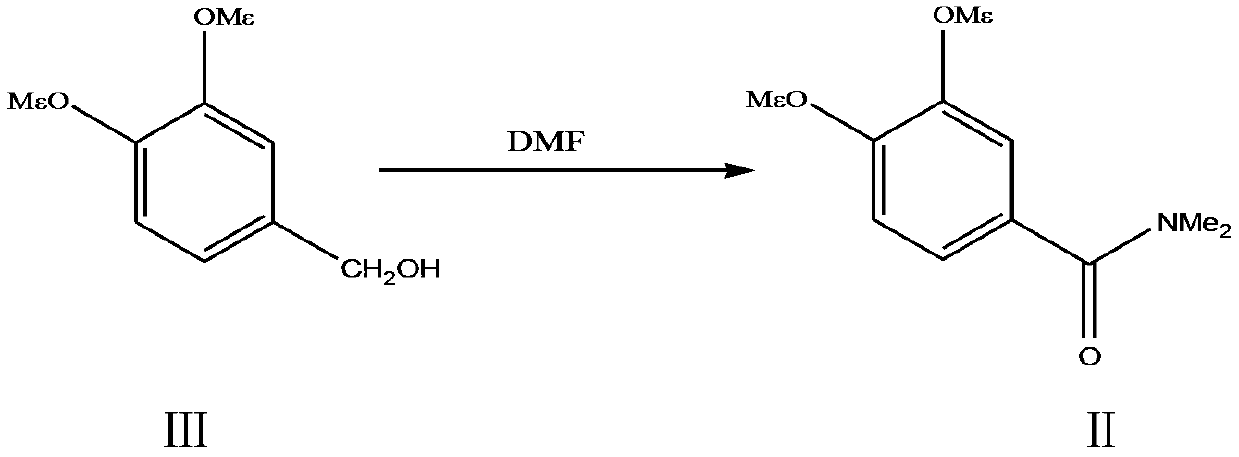
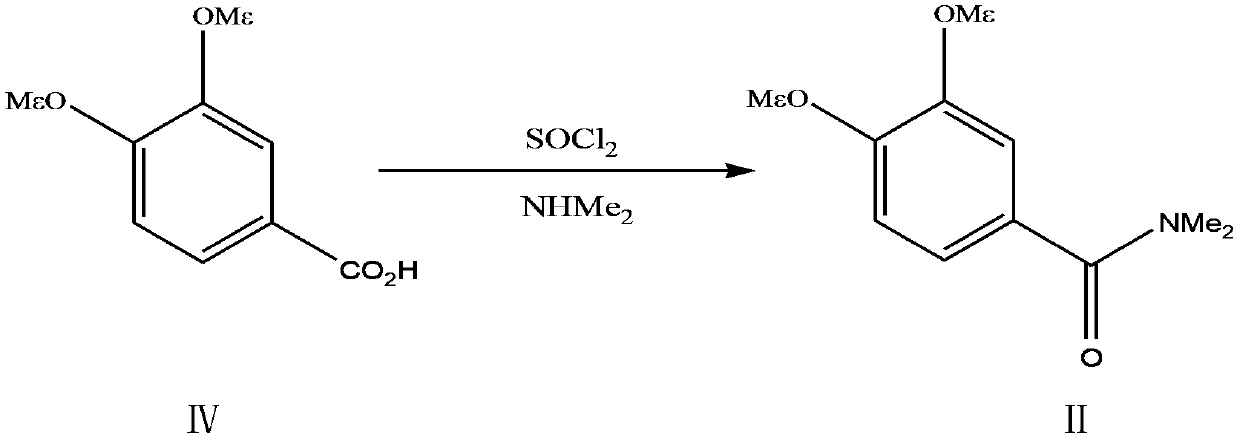
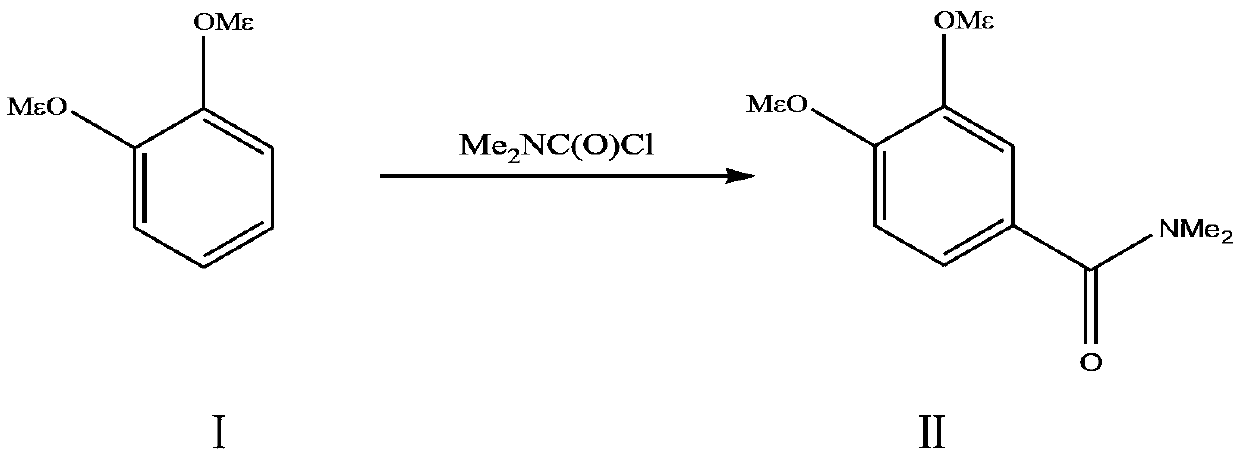
A kind of preparation method of 3,4-dimethoxy-n,n-dimethylbenzamide
A technology of dimethylbenzamide and dimethoxybenzene is applied in the field of preparation of chemical intermediate 3,4-dimethoxy-N,N-dimethylbenzamide, and can solve potential safety hazards, The reaction solvent is flammable and explosive, and the reaction yield is only low, so as to achieve the effects of low cost, easy industrialization and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] In the reaction flask, add 138.2g (1.0mol) of 1,2-dimethoxybenzene, 900g of chlorobenzene, cool down to 5°C, add 200.0g of anhydrous aluminum chloride under stirring, add dropwise N,N-dimethoxybenzene Carbamoyl chloride 160.0 g. Insulate and react at 30-50°C for 20 hours. After the reaction is completed, treat with hydrochloric acid. The obtained organic phase is washed twice with 500ml of water. The organic phase is evaporated to dryness. The obtained material is dissolved with 400g of dimethyl carbonate, and then cooled to 0°C and stirred. After 3 hours of crystallization, the solid was obtained by suction filtration, and the weight was 168.4g after drying. The HPLC content: 98.6%, and the yield was 80.5%. 1 HNMR (CDCl 3 , 400MHz) Characterization: δ2.90 (s, 6H, N-CH 3 ), δ3.71 (s, 3H, -OCH 3 ), δ3.75 (s, 3H, -OCH 3 ), δ6.86 (d, 1H, benzene ring-H), δ7.35 (s, 1H, benzene ring-H), δ7.43 (d, 1H, benzene ring-H).
Embodiment 2
[0027] In the reaction flask, add 138.2g (1.0mol) of 1,2-dimethoxybenzene and 700g of chlorobenzene, cool down to 5°C, add 170.0g of anhydrous aluminum chloride under stirring, add dropwise N,N-dimethoxybenzene Carbamoyl chloride 140.0 g. Insulate and react at 30-50°C for 15h, after the reaction is completed, treat with hydrochloric acid, wash the obtained organic phase twice with 500ml of water, evaporate the organic phase to dryness, dissolve the obtained material with 450g of dimethyl carbonate, and then cool down to 0°C and stir After 3 hours of crystallization, the solid was obtained by suction filtration, and the weight was 163.6g after drying. The HPLC content: 98.5%, and the yield was 78.2%. 1 HNMR (CDCl 3 , 400MHz) Characterization: δ2.90 (s, 6H, N-CH 3 ), δ3.71 (s, 3H, -OCH 3 ), δ3.75 (s, 3H, -OCH 3 ), δ6.86 (d, 1H, benzene ring-H), δ7.35 (s, 1H, benzene ring-H), δ7.43 (d, 1H, benzene ring-H).
Embodiment 3
[0029] In the reaction flask, add 138.2g (1.0mol) of 1,2-dimethoxybenzene, 550g of chlorobenzene, cool down to 5°C, add 160.0g of anhydrous aluminum chloride under stirring, add dropwise N,N-dimethoxybenzene Carbamoyl chloride 130.0 g. Insulate and react at 30-50°C for 15 hours, after the reaction is completed, treat with hydrochloric acid, wash the obtained organic phase twice with 500ml of water, evaporate the organic phase to dryness, dissolve the obtained material with 500g of dimethyl carbonate, and then cool down to 0°C and stir After 3 hours of crystallization, the solid was obtained by suction filtration, and the weight was 159.0 g after drying. The HPLC content: 98.5%, and the yield was 76.0%. 1 HNMR (CDCl 3 , 400MHz) Characterization: δ2.90 (s, 6H, N-CH 3 ), δ3.71 (s, 3H, -OCH 3 ), δ3.75 (s, 3H, -OCH 3 ), δ6.86 (d, 1H, benzene ring-H), δ7.35 (s, 1H, benzene ring-H), δ7.43 (d, 1H, benzene ring-H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com