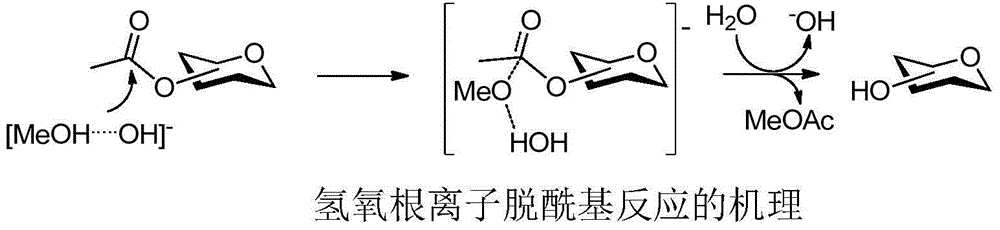
Method for deacylation with hydroxyl ion type alkali as catalyst
A deacylation and hydroxide technology, applied in chemical instruments and methods, organic chemistry, sugar derivatives, etc., can solve problems such as inconvenience, and achieve the effects of simple operation, mild reaction and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0019] Example 1 reaction solvent is methyl alcohol, and catalyzer is sodium hydroxide:
[0020] Weigh 10 g of pentaacetylated glucose, dissolve it in 100 ml of normal methanol, and add 20 mg of sodium hydroxide. The reaction was stirred at room temperature for one hour. After adding 300 mg of hydrogen ion acidic resin for complete neutralization to neutrality, the resin was filtered off, and the solvent was directly evaporated to dryness under reduced pressure to obtain 4.5 g of white powder, which was shown to be pure glucose by NMR analysis, with a yield of 97.5%. Its NMR spectrum data are: 1 H NMR (400MHz,D 2 O):5.23(1H,d,H-1),3.81-3.87(2H,m,H-5,H-6b),3.73-3.79(1H,m,H-6a),3.72(1H,dd, H-3), 3.54(1H, dd, H-2), 3.42(1H, t, H-4).
example 2
[0021] Example 2 reaction solvent is methyl alcohol, and catalyzer is potassium hydroxide:
[0022] Weigh 10 g of pentaacetylated glucose, dissolve it in 100 ml of normal methanol, and add 30 mg of potassium hydroxide. The reaction was stirred at room temperature for one hour. Add 300 mg of hydrogen ion acidic resin to neutralize completely to neutrality, filter off the resin, and directly evaporate the solvent under reduced pressure to obtain 4.55 g of pure glucose in the form of white powder, with a yield of 98.6%.
example 3
[0023] Example 3 sodium hydroxide is catalyst desulfurization acetyl group:
[0024] Weigh 10 g of tetraacetylated mannose protected by the 1-thioacetyl group, dissolve it in 100 ml of ordinary methanol, and add 1.1 g of sodium hydroxide (0.2 mol / mol acyl group). The reaction was stirred at room temperature for one hour. After adding about 1 gram of hydrogen ion acidic resin for complete neutralization to neutrality, the resin was filtered off, and the solvent was directly evaporated to dryness under reduced pressure to obtain 4.8 grams of white powder, which was shown to be pure 1-mercaptomannose by nuclear magnetic analysis, with a yield of 99%. Its NMR spectrum data are: 1 H NMR (400MHz,MeOD):5.51(1H,d,H-1),4.05(1H,dd,H-3),3.92(1H,dd,H-2),3.69-3.85(3H,m,H -5,H-6,H-6'),3.65(1H,t,H-4),2.34(1H,d,SH).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com