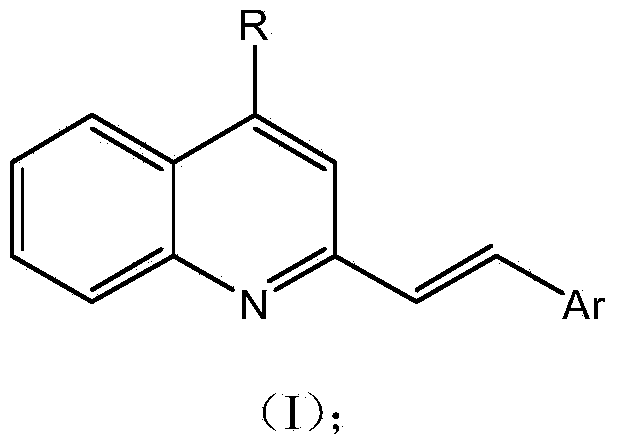
4-substituted-2-arylvinyl quinoline derivative, and preparation method and application thereof
A technology of arylvinylquinoline and its derivatives, which is applied in drug combinations, active ingredients of heterocyclic compounds, muscular system diseases, etc., can solve the problems of complex pathogenesis and mutual influence of Alzheimer's disease, and achieve simple preparation methods , good Aβ aggregation, easy synthesis effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
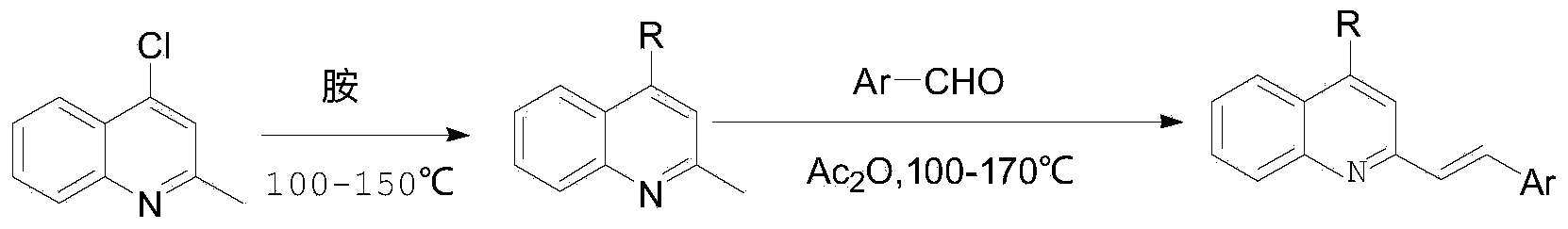
Examples
Embodiment 1
[0034] Embodiment 1: the synthesis of 4-methylpiperazinyl-2-methylquinoline (compound 3a)
[0035] Put 5g of 4-chloro-2-methylquinoline in a 50mL single-necked bottle, add 20mL of methylpiperazine and 0.6g of p-toluenesulfonic acid in sequence, heat to reflux, TLC traces that the reaction is complete (about 10h), after cooling Add 50mL of water, adjust the pH value to be alkaline with aqueous sodium hydroxide solution, extract three times with 50ml, 50ml, and 50ml of dichloromethane, combine the organic phases, wash once with 40mL of water, spin dry the organic layer, and dichloromethane / methanol (volume Purification by silica gel chromatography (ratio 100 / 1) as eluent afforded 3a 5.1 g as a pale yellow oil. Yield 75%; 1 H NMR (400MHz, CDCl 3 )δ7.98(t, J=8Hz, 2H), 7.62(t, J=8Hz, 1H), 7.42(t, J=8Hz, 1H), 6.74(s, 1H), 3.27(s, 4H), 2.73(s,4H),2.69(s,3H),2.43(s,3H).
[0036]
Embodiment 2
[0037] Embodiment 2: the synthesis of compound 3b
[0038] The synthesis method is the same as in Example 1; the difference is that piperidine is used instead of methylpiperazine, and dichloromethane / petroleum ether (volume ratio 1 / 1) is purified by silica gel chromatography as an eluent to obtain a light yellow oil 3b. The yield is 72%; 1 H NMR (400MHz, CDCl 3 )δ7.95(d,2H),7.59(t,J=8Hz,1H),7.40(t,J=8Hz,1H),6.71(s,1H),3.15(s,1H),2.68(d, J=9.9Hz,3H),1.84(s,1H).
[0039]
Embodiment 3
[0040] Embodiment 3: the synthesis of compound 3c
[0041] The synthesis method was the same as in Example 1; the difference was that p-methoxyaniline was used instead of methylpiperazine, and the obtained crude product was recrystallized from ethanol to obtain 3c as a white solid. The yield is 82.1%; 1 H NMR (400MHz, CDCl 3 ): δ7.97(d, J=8.4Hz, 1H), 7.86(d, J=8.3Hz, 1H), 7.65(t, J=7.6Hz, 1H), 7.44(t, J=7.6Hz, 1H ),7.24(d,J=8.9Hz,2H),6.98(d,J=8.8Hz,2H),6.59(s,1H),6.51(s,1H),3.86(s,3H),2.54(s ,3H).
[0042]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com