New fosfomycin derivatives and medicinal use thereof
The technology of a derivative, fosfomycin, is applied in the field of new fosfomycin derivatives and their medical applications, which can solve the problems of high water solubility of fosfomycin, short half-life of fosfomycin, and high concentration of kidney distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
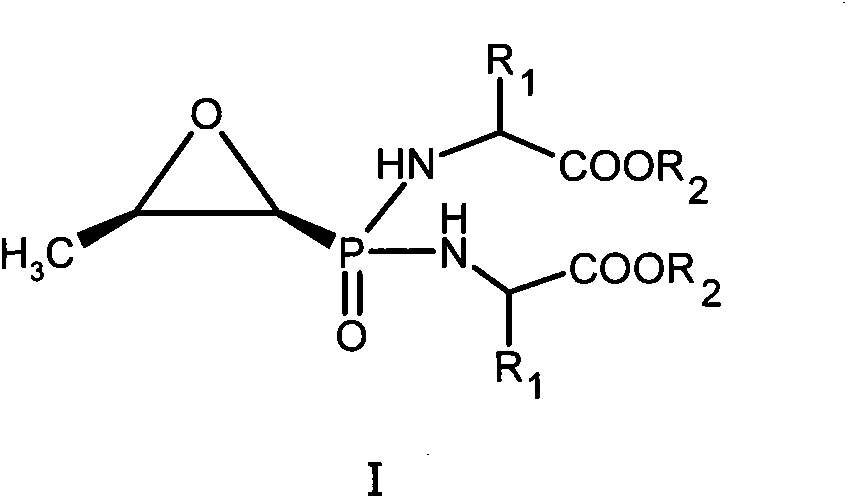
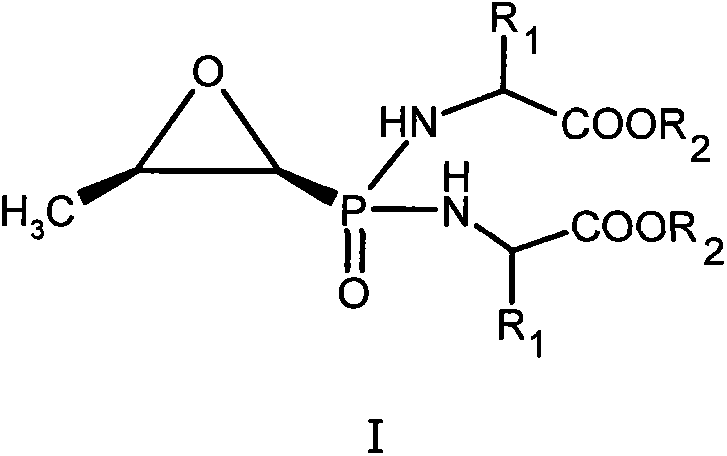
[0018] Example 1 P, P-bis((S)-alanine propyl ester)-N-fosfomycin (I 1 )Synthesis
[0019]
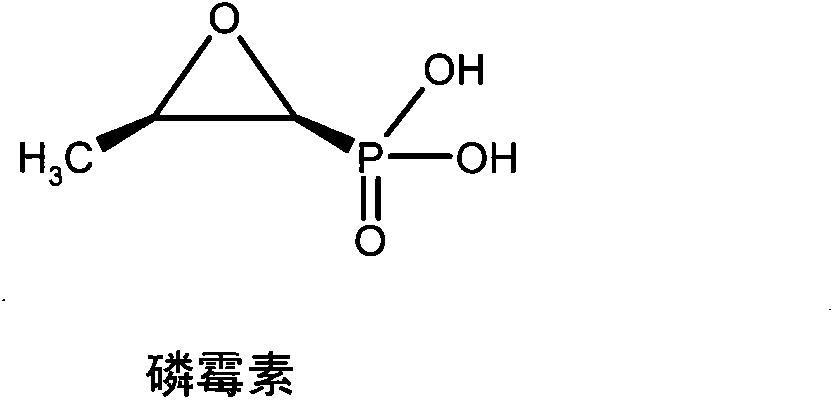
[0020] In 100ml anhydrous pyridine, add 1.16g (6.4mmol) fosfomycin, 6.4g (38.4mmol) (S)-alanine-O-propyl ester hydrochloride, 10.6ml triethylamine, pass N 2 , Stir at 60°C. Then add 11.8 g of 2,2’-dithiodipyridine and 9.8 g of triphenylphosphine dissolved in 50 ml of anhydrous pyridine, in N 2 The reaction was stirred at 60°C for 16h under protection. The reaction solution was evaporated to dryness under reduced pressure, and the residue was dissolved in 100ml of dichloromethane, and 10% Na 2 CO 3 Wash with 1N hydrochloric acid and saturated brine, dry the organic layer with anhydrous sodium sulfate, filter off the desiccant, and separate the residue with silica gel column chromatography, eluting with dichloromethane: petroleum ether (5:5), and collect The required components are evaporated to dryness under reduced pressure to obtain I 1 0.58 grams. Elemental analysis (%), theoretical val...
Embodiment 2
[0021] Example 2 P, P-bis((S)-alanine methyl ester)-N-fosfomycin (I 2 )Synthesis
[0022]
[0023] Refer to the method of Example 1, replace (S)-alanine-O-propyl ester hydrochloride with (S)-alanine-O-methyl ester hydrochloride, and react with fosfomycin sodium to obtain I 2 , The yield is 24%. Elemental analysis (%), theoretical value: C42.86 H6.87 N9.09; experimental value: C42.76 H6.92 N8.97.
Embodiment 3
[0024] Example 3 P, P-bis((S)-alanine ethyl ester)-N-fosfomycin (I 3 )Synthesis
[0025]
[0026] Refer to the method of Example 1, replace (S)-alanine-O-propyl ester hydrochloride with (S)-alanine-O-ethyl ester hydrochloride, and react with fosfomycin sodium to obtain I 3 , The yield is 30%. Elemental analysis (%), theoretical value: C46.43 H7.49 N8.33; experimental value: C46.60 H7.28 N8.39.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com