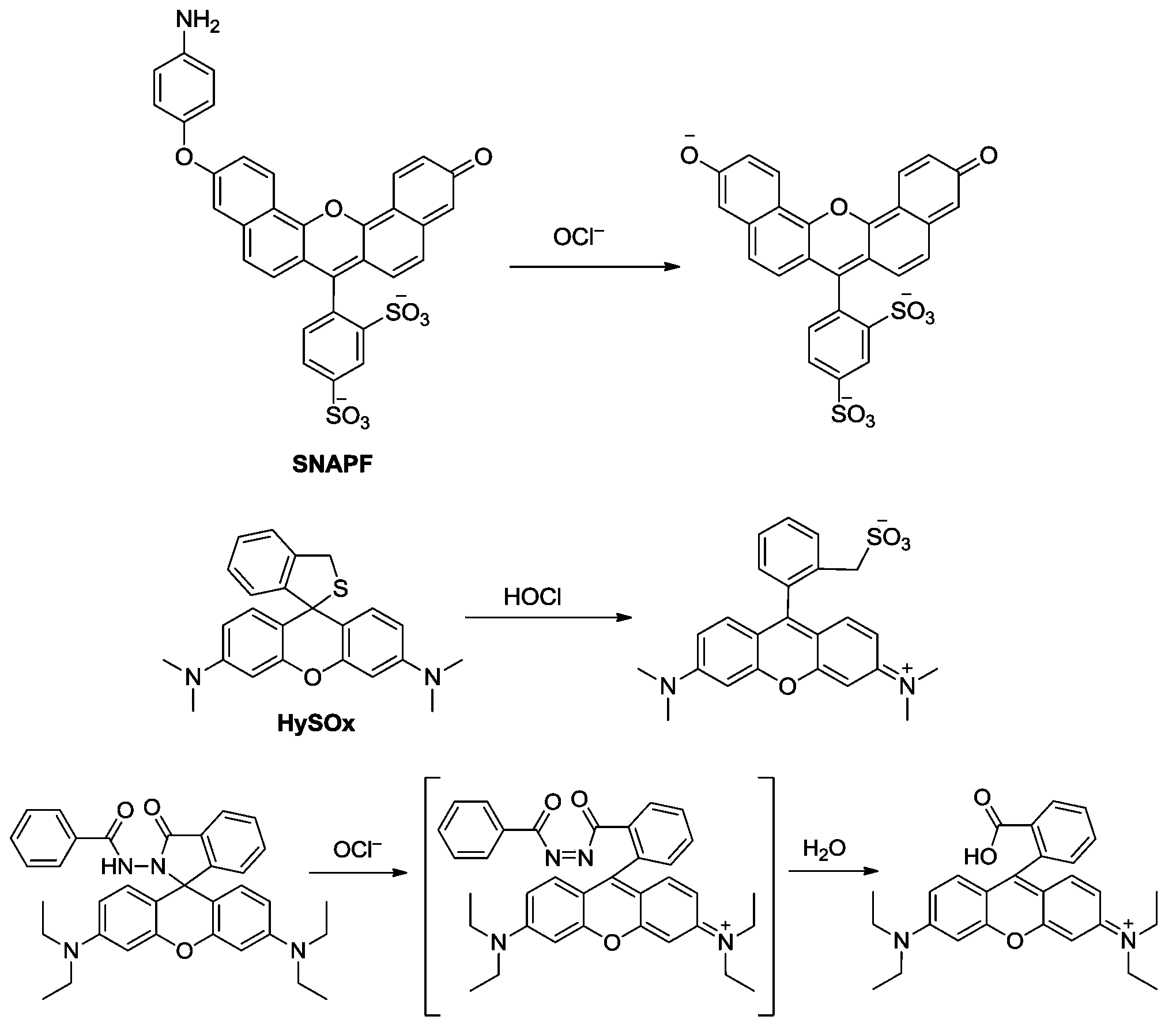
Fluorescent probe for ratio detection of hypochloric acid and application thereof in biological systems
A technology for detecting hypochlorous acid and fluorescent probes, which is applied in the field of fluorescent probes and can solve the problem that the probes do not have biological system detection.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
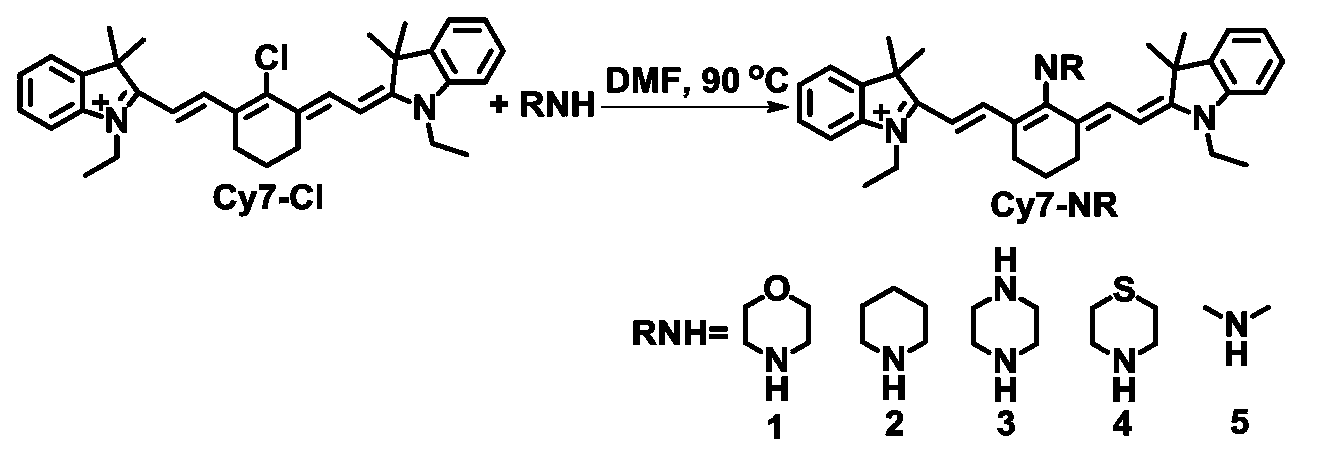
[0037] Example 1 (synthesis and properties of Cy7-NR1):
[0038] Synthesis of Cy7-NR1: Under nitrogen, 0.60g Cy7-Cl (available for purchase) and 1.07g morpholine were dissolved in 25ml N,N-dimethylformamide, heated to 90°C for 3 hours. The solvent was evaporated under vacuum, and the obtained solid was purified by column chromatography to obtain the target compound Cy7-NR1.
[0039] Cy7-NR1: 1 H NMR (400MHz, CDCl 3 )δ(ppm):7.77(d,J=12Hz,2H),7.37-7.32(m,4H),7.16(t,J=8Hz,2H),7.03(d,J=8Hz,2H),5.93( d,J=16Hz,2H),4.11-4.06(m,4H),3.98(t,J=4Hz,4H),3.72(t,J=4Hz,4H),2.56(t,J=6Hz,4H) ,1.88(t,J=6Hz,2H),1.69(s,12H),1.44(t,J=6Hz,6H). 13 C NMR (400MHz, CDCl 3 )δ (ppm): 171.78, 168.91, 142.16, 141.59, 140.39, 128.65, 125.31, 123.97, 122.10, 109.57, 96.91, 68.31, 55.01, 48.30, 39.05, 28.84, 25.31, 21.069,
[0040] HRMS: m / z C 38 h 48 N 3 o + Calcd 562.3797, found 562.2762.
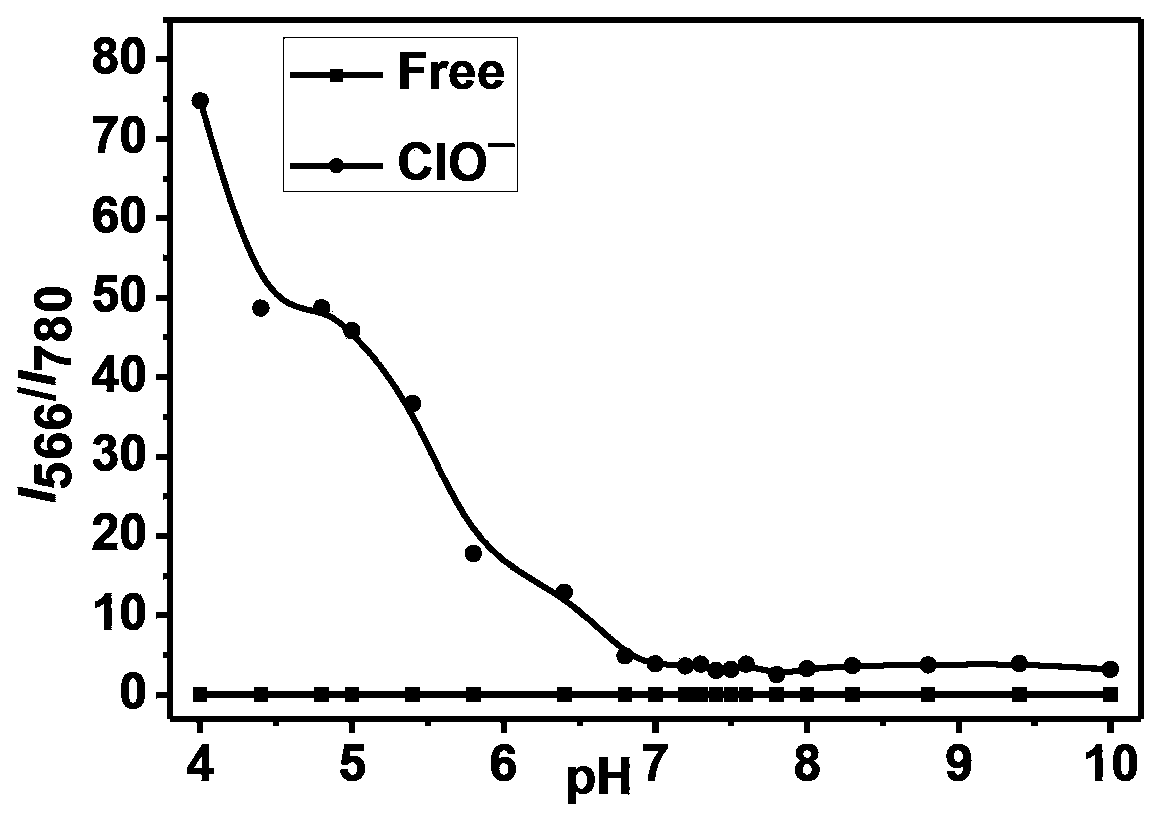
[0041] The effect of pH on fluorescence of Cy7-NR1 solution: pH was controlled by PBS buffer solution. Add ...
Embodiment 2
[0047] Embodiment 2 (the synthesis of Cy7-NR2 and the selectivity to hypochlorous acid):
[0048] Synthesis of Cy7-NR2: Under nitrogen, 0.60g Cy7-Cl (available for purchase) and 10 equivalents of piperidine were dissolved in 25ml N,N-dimethylformamide, heated to 90°C for 3 hours. The solvent was evaporated under vacuum, and the obtained solid was purified by column chromatography to obtain the target compound Cy7-NR2.
[0049] Cy7-NR2: 1 H NMR (400MHz, CDCl 3 )δ(ppm):7.55(d,J=16Hz,2H),7.34-7.27(m,4H),7.11(t,J=8Hz,2H),6.95(d,J=8Hz,2H),5.75( d,J=12Hz,2H),3.97(t,J=6Hz,4H),3.67-3.64(m,4H),2.50(t,J=6Hz,4H),1.92-1.82(m,8H),1.66 (s,12H),1.38(t,J=8Hz,6H).HRMS:m / z C 39 h 50 N 3 + Calcd 560.4005, found 560.3985.
[0050] Selectivity of Cy7-NR2 to hypochlorous acid: add 5.0μMCy7-NR2 to a 10ml colorimetric tube, then add 10ml of PBS (pH=7.4) with a concentration of 20mM, shake well, and then add various substances to be tested (each For the amount of the measured substance, see t...
Embodiment 3
[0051] Embodiment 3 (the synthesis of Cy7-NR3 and the selectivity to hypochlorous acid):
[0052] Synthesis of Cy7-NR3: Under nitrogen, 0.60g Cy7-Cl (available for purchase) and 10 equivalents of piperazine were dissolved in 25ml N,N-dimethylformamide, heated to 90°C for 3 hours. The solvent was evaporated under vacuum, and the obtained solid was purified by column chromatography to obtain the target compound Cy7-NR3.
[0053] Cy7-NR3: 1H NMR (400MHz, CDCl 3 )δ(ppm):7.85(d,J=12Hz,2H),7.42(d,J=8Hz,2H),7.36(t,J=6Hz,2H),7.19(t,J=8Hz,2H), 7.05(d,J=8Hz,2H),5.90(d,J=16Hz,2H),4.05(t,J=8Hz,8H),3.53(s,4H),2.52(t,J=6Hz,4H) ,1.88-1.85(m,2H),1.80(s,12H),1.44(t,J=8Hz,6H).HRMS:m / z C 38 h 49 N 4 + Calcd 561.3957, found 561.3971.
[0054] Selectivity of Cy7-NR3 to hypochlorous acid: add 5.0μMCy7-NR3 to a 10ml colorimetric tube, then add 10ml of PBS (pH=7.4) with a concentration of 20mM, shake well, and then add various substances to be tested (each For the amount of the measured subs...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com