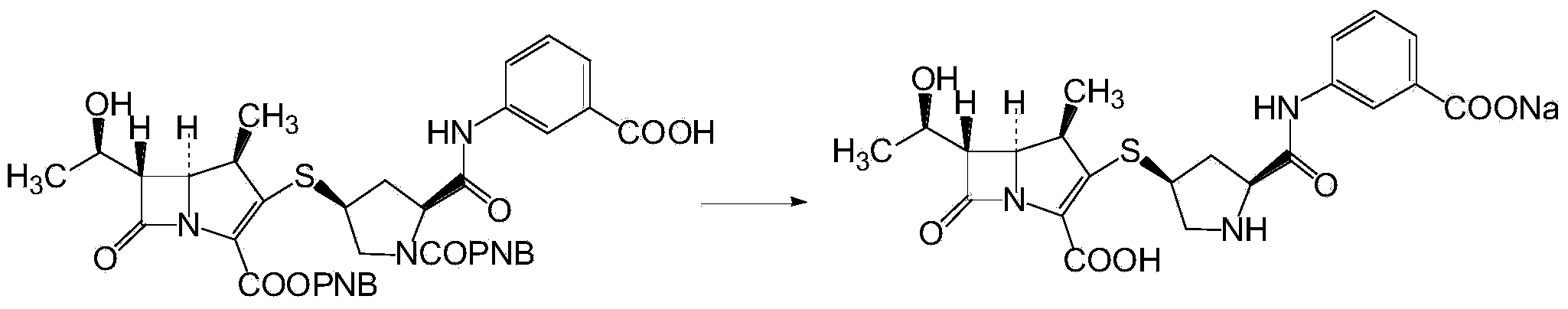
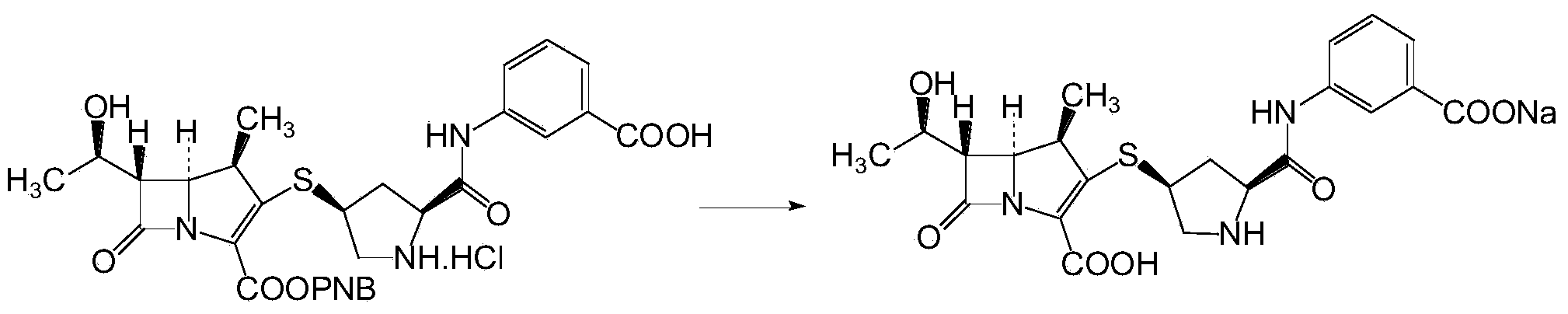
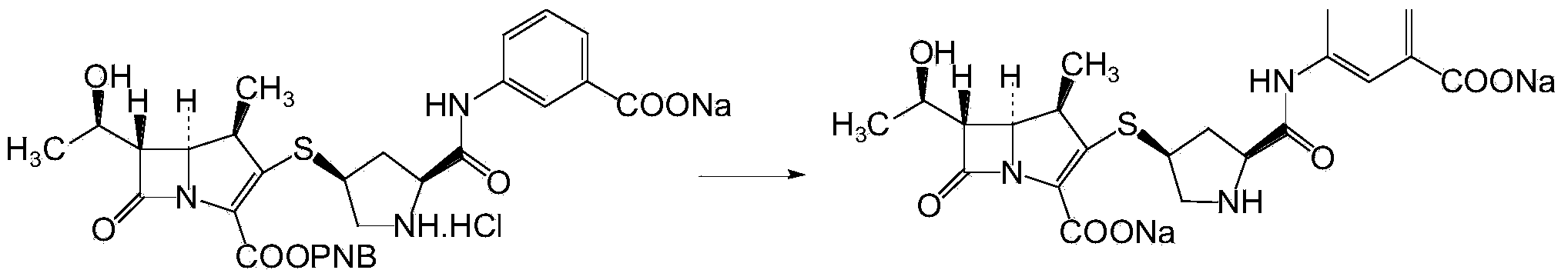
Ertapenem and ertapenem side chain, as well as preparation methods of ertapenem and ertapenem side chains
A technology of ertapenem side chain and ertapenem, applied in the field of antibiotic preparation, can solve the problems of high raw material cost, labor cost, cumbersome operation, and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0123] Synthesis of (2S,4R)-4-hydroxy-1-(((4-nitrobenzoyl)oxy)carbonyl)pyrrolidine-2-carboxylic acid (5):
[0124] Add 12kg of water and 1kg of sodium hydroxide to a 20L reactor, stir to dissolve, cool down to 25-30°C, add 1.5kg of L-hydroxyproline, stir to dissolve, cool down to 0-5°C, and start to control the temperature at 0-5°C A solution of 2.7 kg of p-nitrobenzyl chloroformate and 3 kg of dichloromethane was added dropwise. The dropwise addition time is about 2 hours. After the dropwise addition is completed, keep stirring at about 0-5°C for 3 hours. The detection response is complete. Separate the dichloromethane phase, wash the water phase with 3 kg of dichloromethane, separate the water phase, control the temperature to no more than 15 degrees, adjust the pH value to 2 with concentrated sulfuric acid, cool down to 0°C, filter, wash with water, and dry to obtain the product (2S, 4R)- 4-Hydroxy-1-(((4-nitrobenzoyl)oxy)carbonyl)pyrrolidine-2-carboxylic acid (5) 31.95kg...
Embodiment 2
[0126] Synthesis of 4-nitro(1S,4S)-3-oxo-2-thia-5-azabicyclo[2.2.1]heptane-5-carboxylic anhydride (3):
[0127] Under nitrogen protection, 31.3 g of (2S,4R)-4-hydroxy-1-(((4-nitrobenzyl)oxy)carbonyl)pyrrolidine-2-carboxylic acid (5) was dissolved in 300 mL of dichloromethane, Add 12.1 g of triethylamine, stir to dissolve and cool down to -15°C, add 13.5 g of isopropyl chloroformate dropwise, and stir and react at this temperature for 30 minutes. 16.2 g of triethylamine was added, and 16 g of methanesulfonyl chloride was added dropwise at this temperature, followed by stirring at this temperature for 30 minutes. Add 25.3 g of triethylamine and 100 mL of 10.2 g of sodium sulfide water. React at 5-10°C for 1 hour. After the reaction was completed, wash once with 300 mL of 1N hydrochloric acid, once with 300 mL of saturated brine, and extract once with 200 mL of dichloromethane for combining the aqueous phases. Combined with anhydrous sodium sulfate and dried, filtered, and the...
Embodiment 3
[0129] (2S, 4S)-4-nitrobenzyl-4-mercapto-2-((3-(((4-nitrobenzyl)oxy)carbonyl)phenyl)carbamoyl)pyrrolidine-1- Synthesis of formate (2) (ie: ertapenem side chain III):
[0130] Under nitrogen protection, 20g of 4-nitro(1S,4S)-3-oxo-2-thia-5-azabicyclo[2.2.1]heptane-5-carboxylic anhydride (3) was dissolved in dichloro 140mL of methane was added, 19.5g of 3-aminobenzoic acid p-nitrobenzyl ester was added, the reaction was stirred at room temperature for 7 hours, and the solvent was evaporated to obtain (2S, 4S)-4-nitrobenzyl-4-mercapto-2-((3- (((4-nitrobenzyl)oxy)carbonyl)phenyl)carbamoyl)pyrrolidine-1-carboxylate (2). mp.148-150°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com