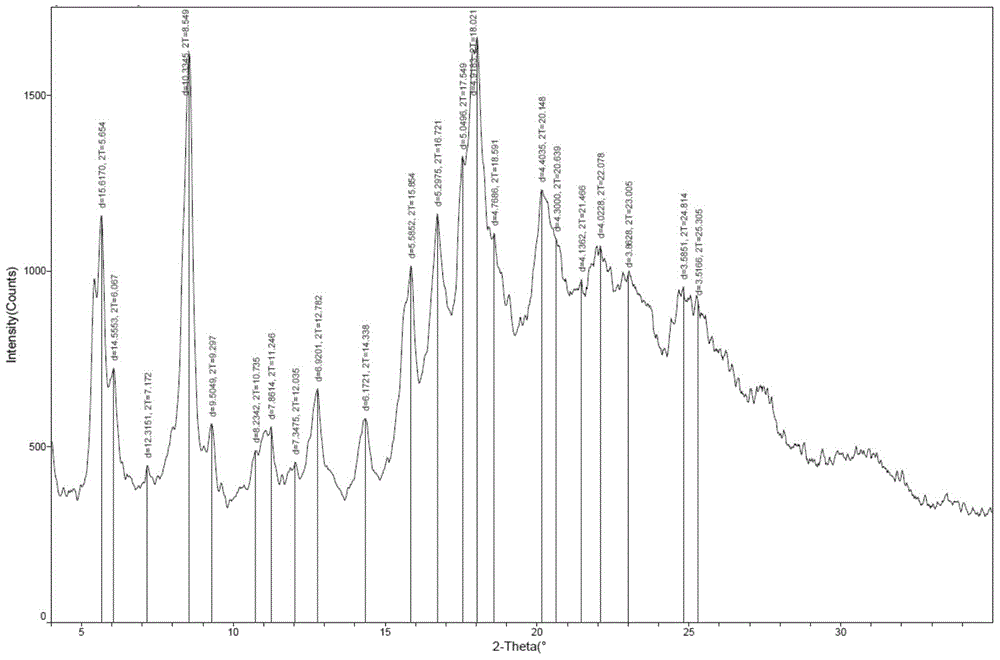
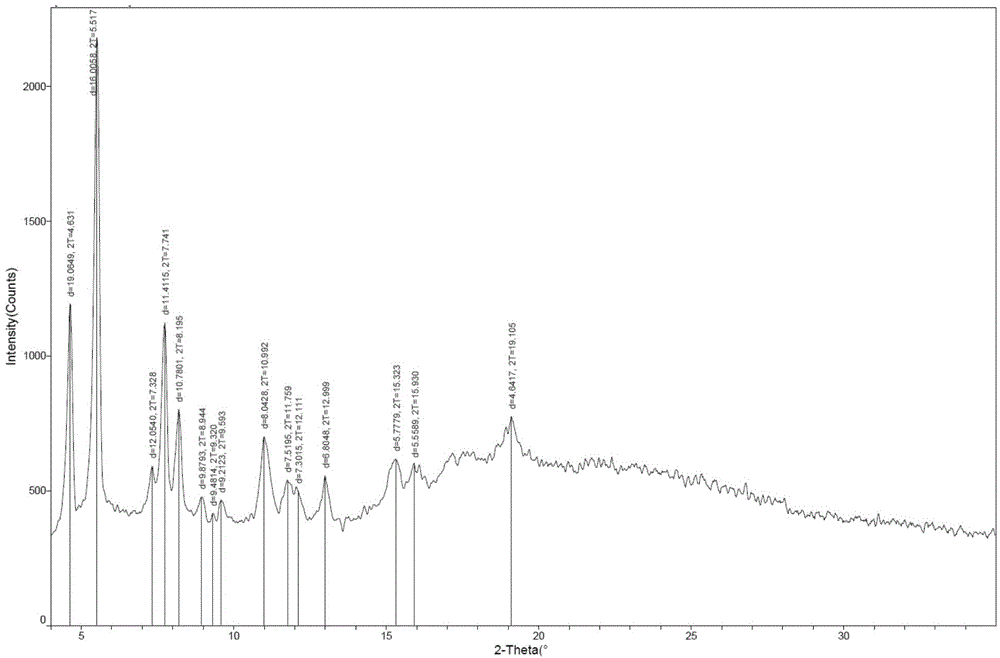
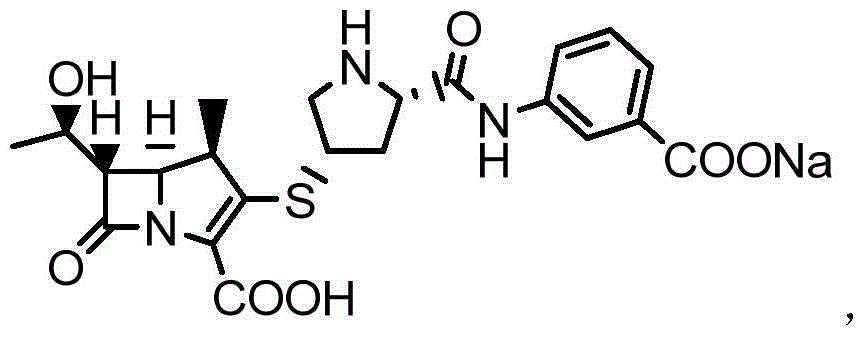
One-pot preparation process of monosodium ertapenem
A preparation process, the technology of ertapenem, which is applied in the preparation of ertapenem monosodium salt and in the field of pharmaceutical synthesis, can solve the problems of poor product purity and excessive heavy metals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1: Preparation of double-protected ertapenem crystal form
[0057] In a dry four-neck flask, put carbapenem core (40.0g, 0.067mol), 60ml of dimethylformamide,
[0058] Under the protection of nitrogen, stir to dissolve and clarify. Cool to -40~-30°C, add diisopropylethylamine (17.4g, 0.135mol) dropwise, and control the temperature at -40~-30°C. After the dropping, ertapenem side chain (31.60 g, 0.071 mol) dissolved in 120 ml of dimethylformamide was added dropwise at -40 to -30°C. After dropping, keep warm at -40~-30°C until the reaction is complete, add 400ml of dichloromethane to the reaction solution, and then wash with potassium dihydrogen phosphate buffer solution with a pH value of 2-10, and the organic layer obtained at -5~-10 Stir and crystallize at ℃ overnight, filter, wash the filter cake with a small amount of dichloromethane, filter dry, and send it to 25 ℃ for vacuum drying to obtain 49.9g of double-protected ertapenem crystal form, the yield is 9...
Embodiment 2
[0059] Example 2: Preparation of Ertapenem Monosodium Salt
[0060] In a dry four-neck flask, add carbapenem nucleus (36.2 g, 0.061 mol) and 110 ml of dimethylformamide, stir to dissolve and clarify under nitrogen protection. Cool to -40~-30°C, add diisopropylethylamine (15.74g, 0.122mol) dropwise, and control the temperature at -40~-30°C. After the dropping, ertapenem side chain (29.0 g, 0.065 mol) dissolved in 110 ml of dimethylformamide was added dropwise at -40 to -30°C. After dropping, keep warm at -40~-30°C until the reaction is complete, add 400ml of dichloromethane to the reaction solution, then wash with potassium dihydrogen phosphate buffer solution with a pH value of 2-10, concentrate the obtained organic layer to dryness, add 300ml THF was stirred to dissolve and clarified, then cooled to about 0-5°C, and 160ml of 5% sodium bicarbonate solution was added dropwise. After dropping, the mixture was poured into a 2000L autoclave, and then 12.0g of 10% palladium carbon...
Embodiment 3
[0061] Example 3: Preparation of Ertapenem Monosodium Salt
[0062] In a dry four-neck flask, add carbapenem nucleus (36.2 g, 0.061 mol) and 60 ml of dimethylformamide, stir to dissolve and clarify under nitrogen protection. Cool to -40~-30°C, add diisopropylethylamine (15.74g, 0.122mol) dropwise, and control the temperature at -40~-30°C. After the dropping, ertapenem side chain (27.16 g, 0.061 mol) dissolved in 60 ml of dimethylformamide was added dropwise at -40 to -30°C. After dropping, keep warm at -40~-30°C until the reaction is complete. Add 350ml of dichloromethane and 50ml of methanol to the reaction solution, then wash with potassium dihydrogen phosphate buffer solution with a pH value of 2-10, and concentrate the obtained organic layer to dryness. , add 300ml THF, stir to dissolve and clarify, then cool to about 0-5°C, add 400ml of 2% sodium bicarbonate solution dropwise. The kettle was replaced with nitrogen, and then replaced with hydrogen. After the replacement,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com