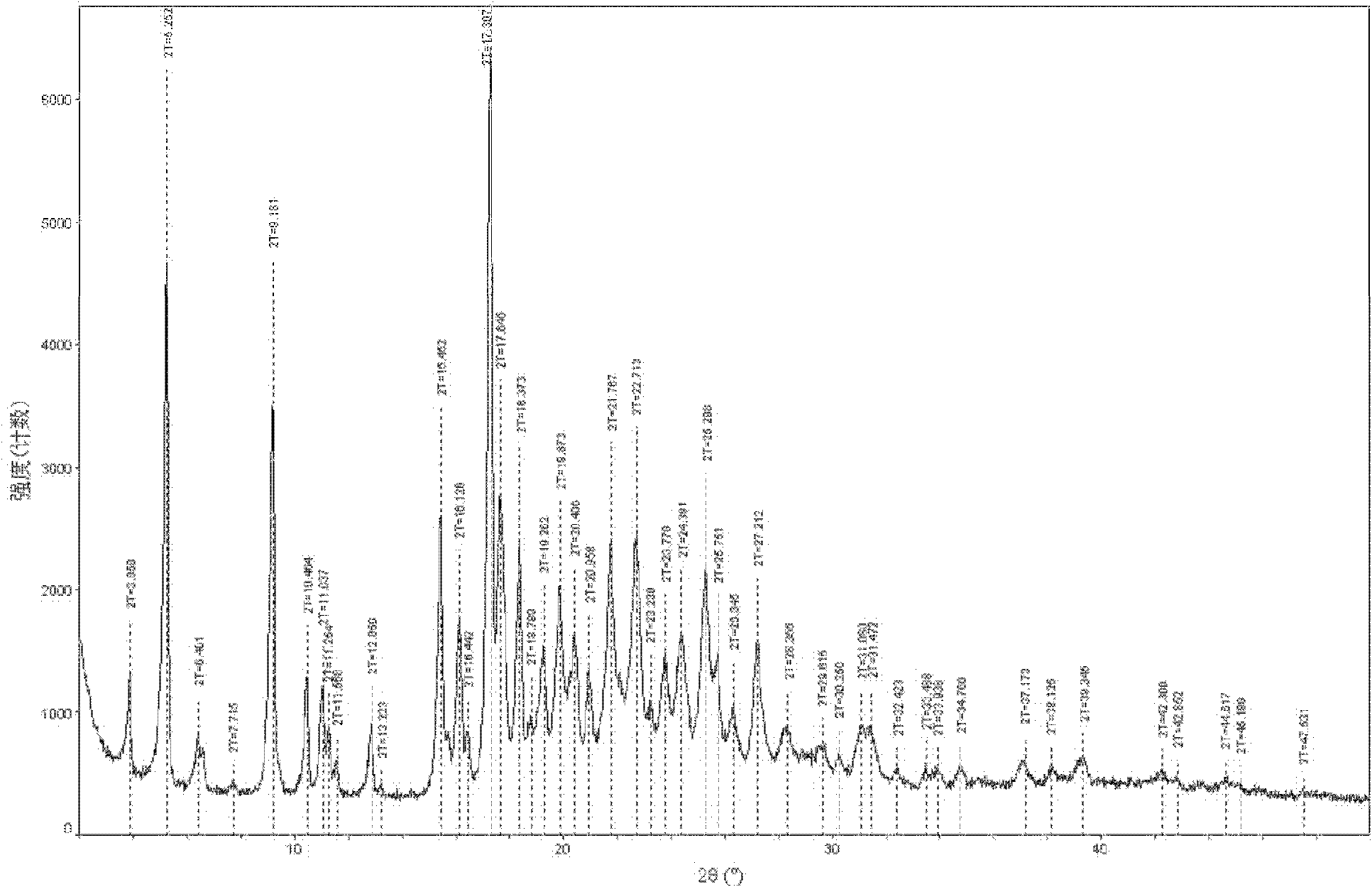
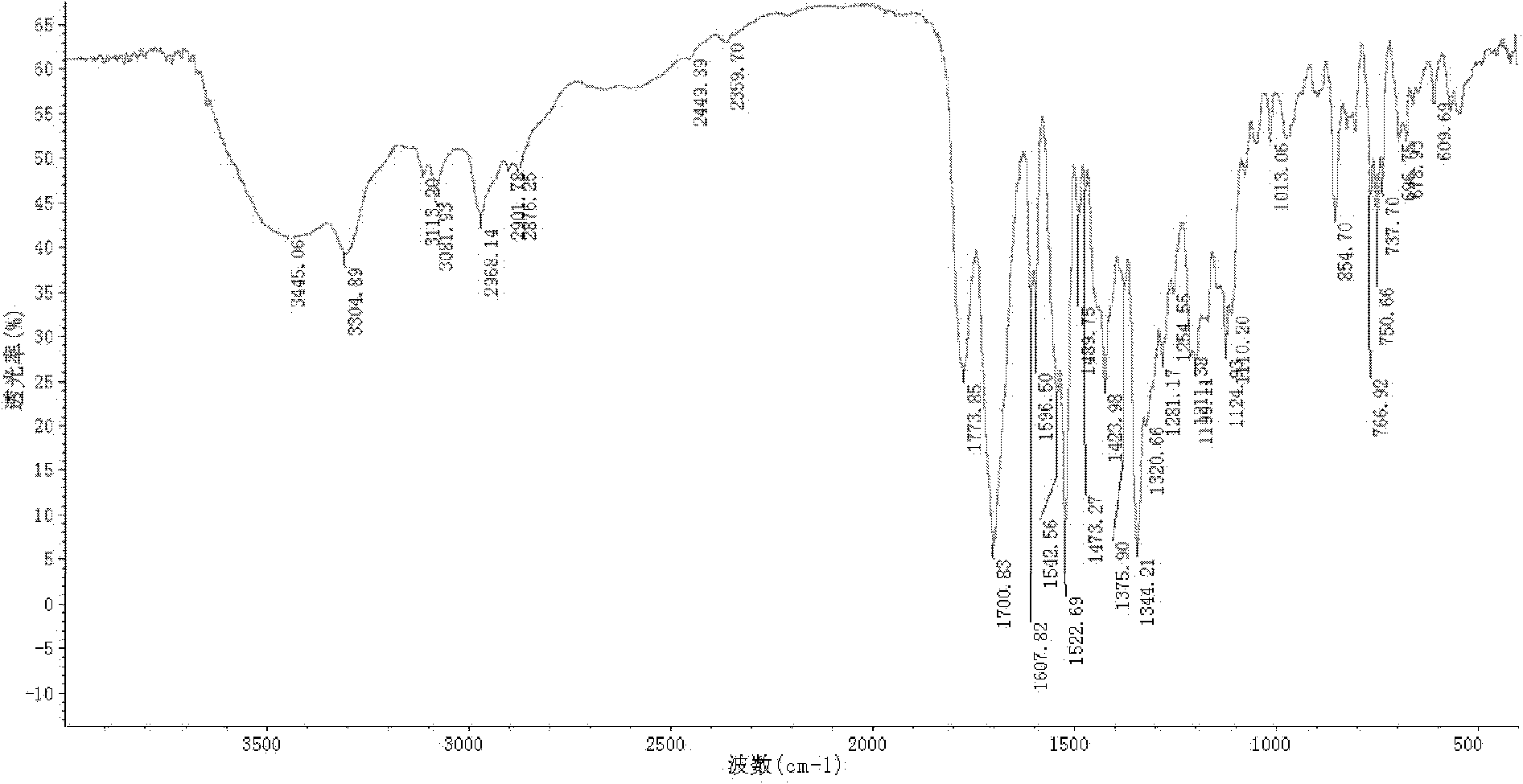
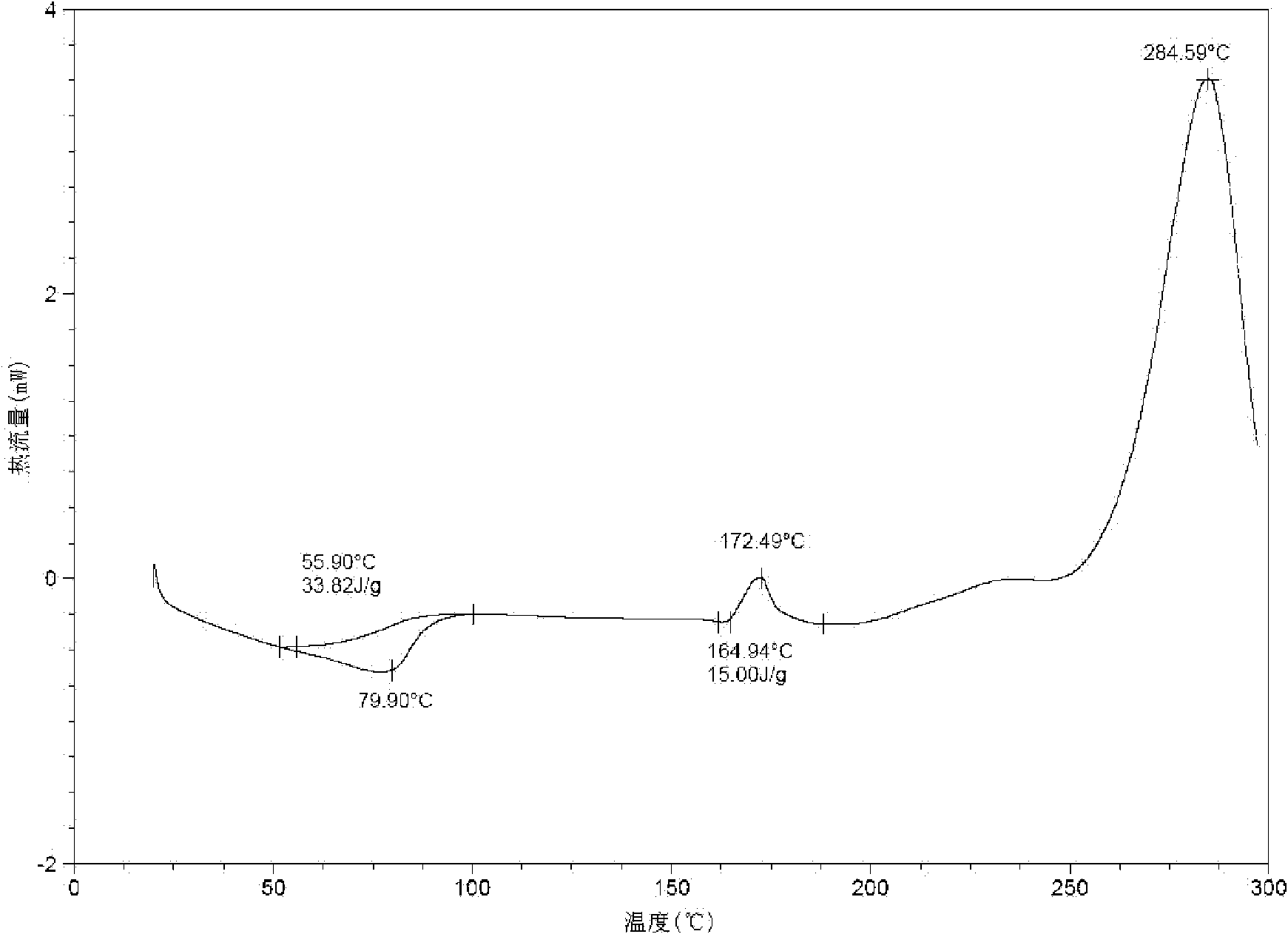
Dual-protection ertapenem crystal and preparation method thereof
A technology for ertapenem and ertapenem side chains, applied in the field of a double-protected ertapenem crystal and a preparation method thereof, can solve the unreported amorphous stability data of double-protected ertapenem , Unable to obtain stable double-protected ertapenem, unreported double-protected ertapenem purification method, etc., to achieve the effect of easy to achieve large-scale, cheap raw materials, and practical promotion value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054]Under the protection of argon, dissolve 25.0g (42.0mmol) of carbapenem core MAP and 19.0g (42.6mmol) of ertapenem side chain in 200ml DMF, then cool down to 0-5°C; DMF (35ml) solution of 13.4g (133mmol) of isopropylamine. After the reaction is completed, the reaction solution is poured into a solution of potassium dihydrogenphosphate buffer solution (pH=4.0, 1500ml) and ethyl acetate precooled to 10°C. In the mixed solvent formed by 500ml, extract and separate layers, then add 200ml of the above buffer solution to the organic layer to wash; Add 180ml of n-hexane dropwise, filter after dropping, wash the filter cake with 60ml of a mixed solvent (volume ratio 1:1) of ethyl acetate and n-hexane with a volume ratio of 1:1, and finally dry it under vacuum at 20°C to obtain a white solid 30.7 g of double-protected ertapenem, the molar yield based on side chains was 92%, and the HPLC purity was 98.5%.
Embodiment 2
[0056] Under the protection of argon, dissolve 25.0g (42.0mmol) of carbapenem core MAP and 18.9g (42.4mmol) of ertapenem side chain in 200ml DMF, then cool down to 0-5°C; DMF (35ml) solution of 8.5g (84mmol) of isopropylamine. After the reaction is completed, the reaction solution is poured into a solution of dipotassium hydrogen phosphate buffer solution (pH=6.0, 1000ml) and methyl formate, which is pre-cooled to 15°C. In the mixed solvent formed by 1000ml, extract and separate layers, and then add 200ml of the above buffer solution to the organic layer to wash; Add 180ml of n-pentane dropwise, filter after dropping, wash the filter cake with a mixed solvent (1:1 by volume) of methyl formate and n-pentane with a volume ratio of 1:1 with 60ml, and finally dry it in vacuum at 25°C to obtain White solid double-protected ertapenem 30.0g, the molar yield based on the side chain is 90%, and the HPLC purity is 98.0%.
Embodiment 3
[0058] Under the protection of argon, dissolve 25.0g (42.0mmol) of carbapenem core MAP and 19.1g (42.9mmol) of ertapenem side chain in 200ml DMF, then cool down to 0-5°C; 12.7g (126mmol) of isopropylamine in DMF (35ml) solution. After the reaction is completed, the reaction solution is poured into a solution of sodium dihydrogen phosphate buffer (pH = 4.5, 1000ml) and ethyl formate In the mixed solvent formed by 2000ml, extract and separate layers, then add 200ml of the above buffer solution to the organic layer to wash; the organic layer is dried with anhydrous sodium sulfate, filtered, stirred and crystallized at 20-25°C for 6h, and then heated at 20-25°C Add 180ml of n-heptane dropwise, filter after dropping, wash the filter cake with a mixed solvent (1:1 by volume ratio) of ethyl formate and n-heptane with a volume ratio of 1:1 with 60ml, and finally dry it under vacuum at 30°C to obtain 29.5 g of white solid double-protected ertapenem, the molar yield based on side chains...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com