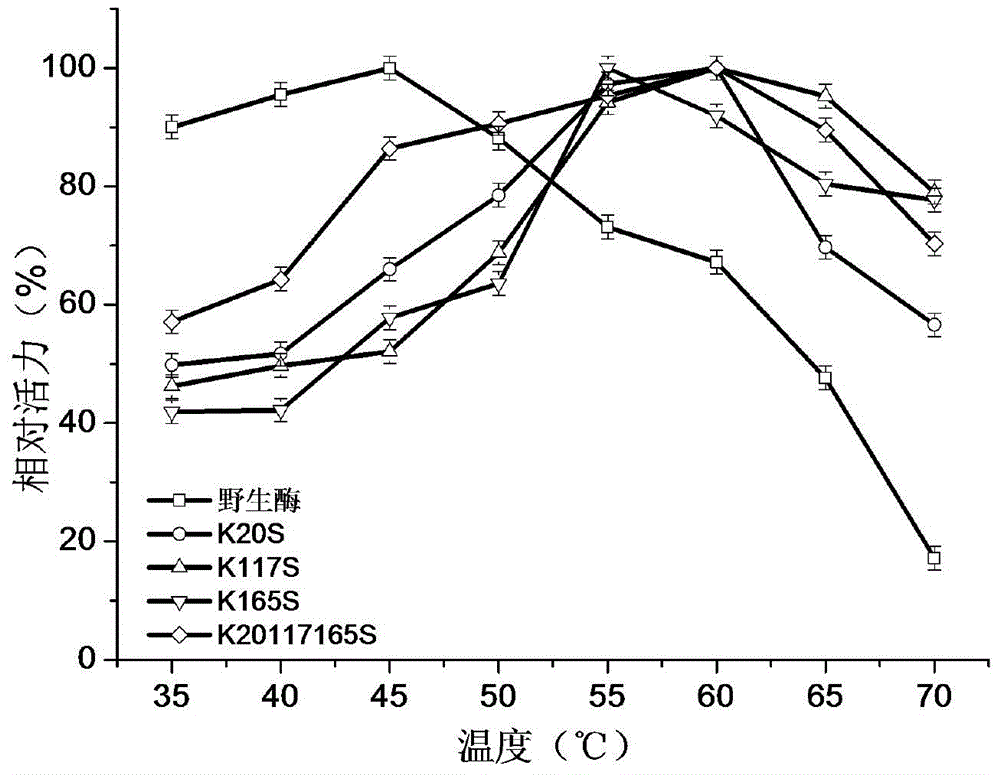
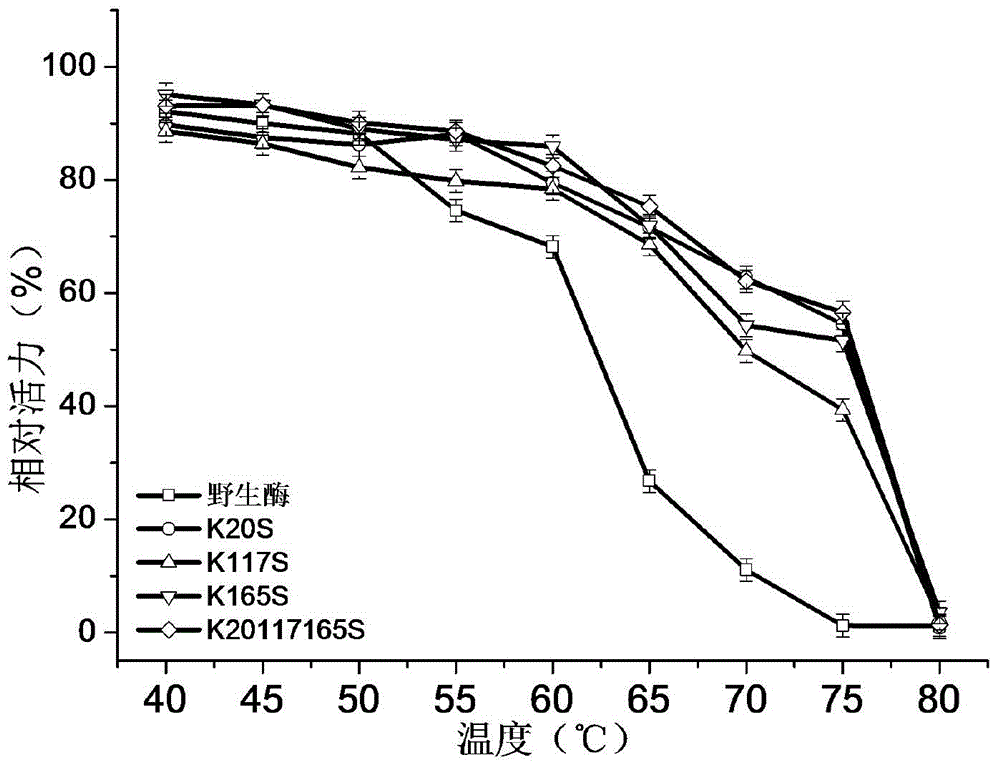
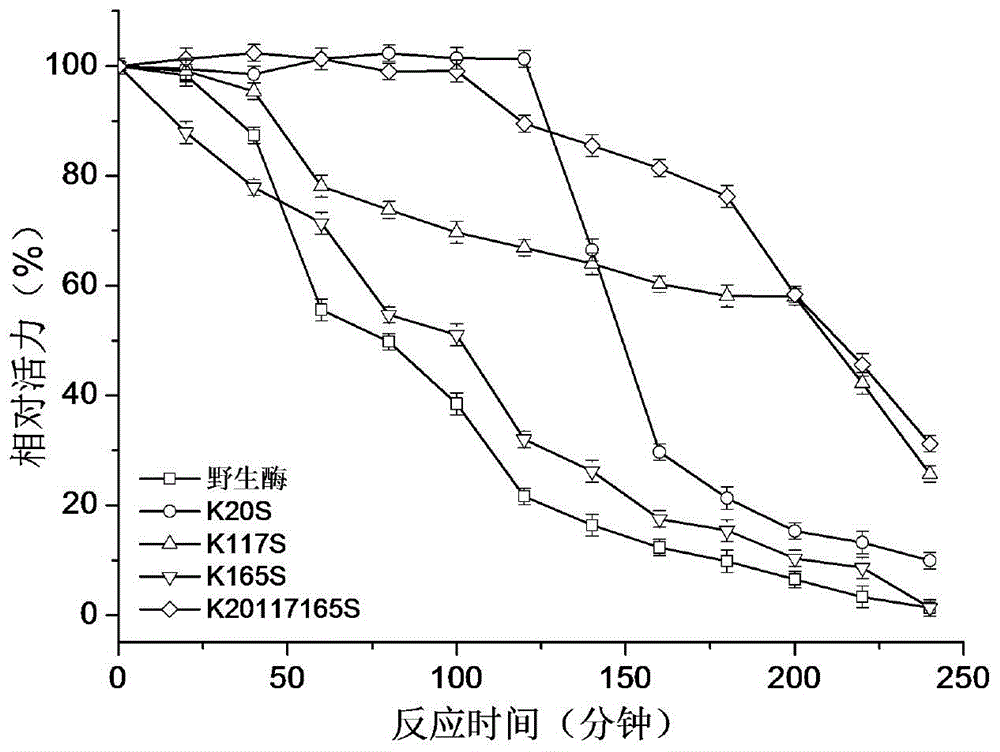
1,3-1,4-Beta-glucanase mutant
A technology of dextranase and mutants, which is applied in the fields of genetic engineering and enzyme engineering, can solve problems such as inability to adapt to industrial applications, and achieve the effect of improving catalytic activity and thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Selection of mutation sites
[0030] Submit the deduced amino acid sequence of β-glucanase to the I-TASSER online server for homology modeling, and input the PDB file obtained from the modeling into Voronoia software to calculate the position of lysine in the structure. It can be seen from Table 1 that in the three-dimensional structure of β-glucanase, the average packing values of all 12 lysines are between 0.47 and 0.62, all of which are on the surface of the protein and have the highest solution accessibility. This means that lysine located on the surface of the protein may have an effect on the thermostability of β-glucanase.
[0031] Table 1 The average packing density of 12 lysines in β-glucanase
[0032]
[0033] Using the genome of Bacillus tequila CGX5-1 as a template, the bglt gene encoding β-glucanase was amplified by PCR, and its nucleotide sequence was shown in SEQ ID NO.9. Using the overlap extension PCR technique and using the expression ve...
Embodiment 2
[0037] Example 2 Preparation of β-glucanase mutants K20S, K117S, K165S and K20S / K117S / K165S
[0038] (1) Site-directed mutation
[0039] 1) Construction of vector pET28a(+)-bglt
[0040] Using the genome of Bacillus tequila CGX5-1 as a template, the bglt gene was amplified by PCR. Primers are as follows:
[0041] Forward primer: 5'-C GGATCC ATGAAACGAGTGTTGCTAATT-3', the underline is the BamHI restriction site,
[0042] Reverse primer: 5'-T CTCGAG gTATTTTTTTGTATAGCGCAC-3', the underline is the XhoI restriction site, and the lowercase letter is the mutation site;
[0043] The PCR reaction system is: 5U / μL rTaq 1μL, 10×rTaq Buffer 5μL, 2.5mM dNTPs 4μL, 100μM forward primer 1μL, 100μM reverse primer 1μL, second-step PCR product 20μL, add double distilled water to make up to 50μL;
[0044] PCR reaction amplification conditions: pre-denaturation at 94°C for 5 minutes; followed by 35 cycles of 94°C for 1min, 56°C for 50s, and 72°C for 50s;
[0045] The PCR amplified product ...
Embodiment 3
[0070] Example 3 Analysis of Enzyme Activity and Protein Concentration
[0071] (1) Enzyme activity assay method:
[0072] 3,5-Dinitrosalicylic acid (DNS) method combined with improved AZO assay method for the determination of β-glucanase activity:
[0073] Enzyme activity definition: 1 mL of enzyme solution under the conditions of 40°C and pH value of 6.5, the amount of hydrolyzing β-glucan per minute to produce glucose reducing substances equivalent to 1 μmol is 1 enzyme activity unit, expressed in U / mL.
[0074] Determination of the enzyme activity of the fermentation broth: after the fermentation broth is centrifuged, the supernatant is diluted to an appropriate multiple to measure its enzyme activity.
[0075]Drawing of glucose standard curve: draw 1% glucose standard solution 2.0, 3.0, 4.0, 5.0, 6.0mL respectively into 50mL volumetric flask, dilute to the mark with distilled water, and make each milliliter contain glucose 200, 400, 600, 800 , 1000, 1200μg dilute standa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific enzyme activity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com