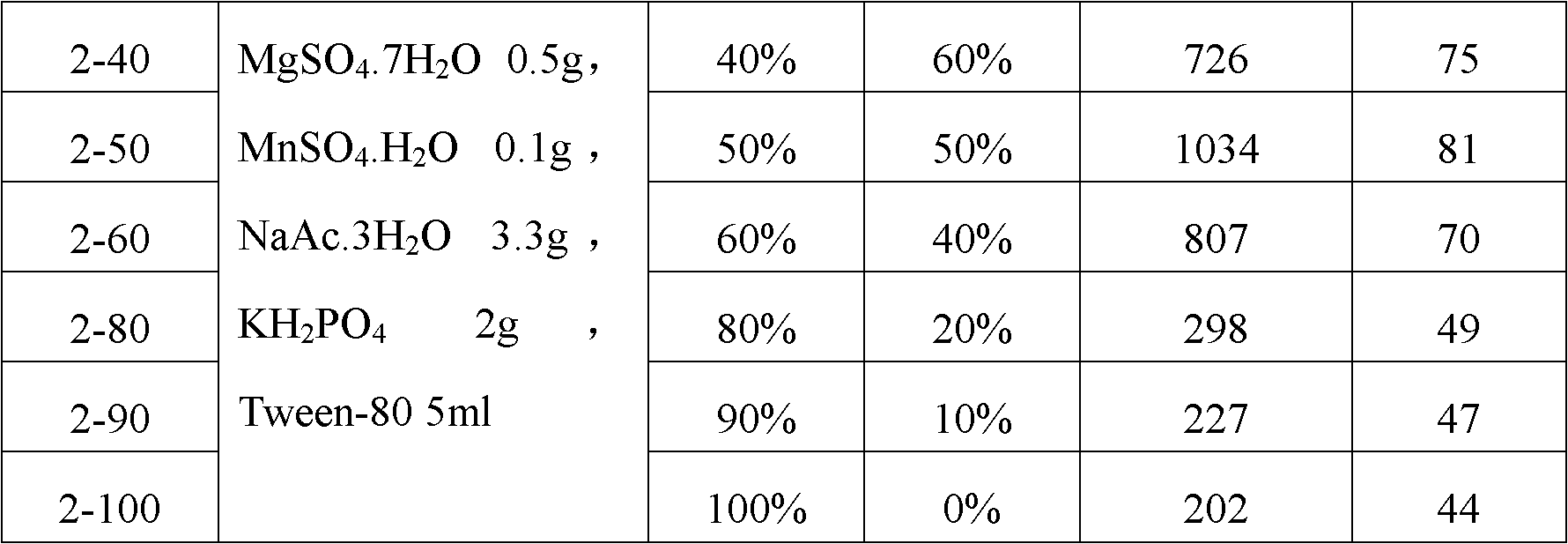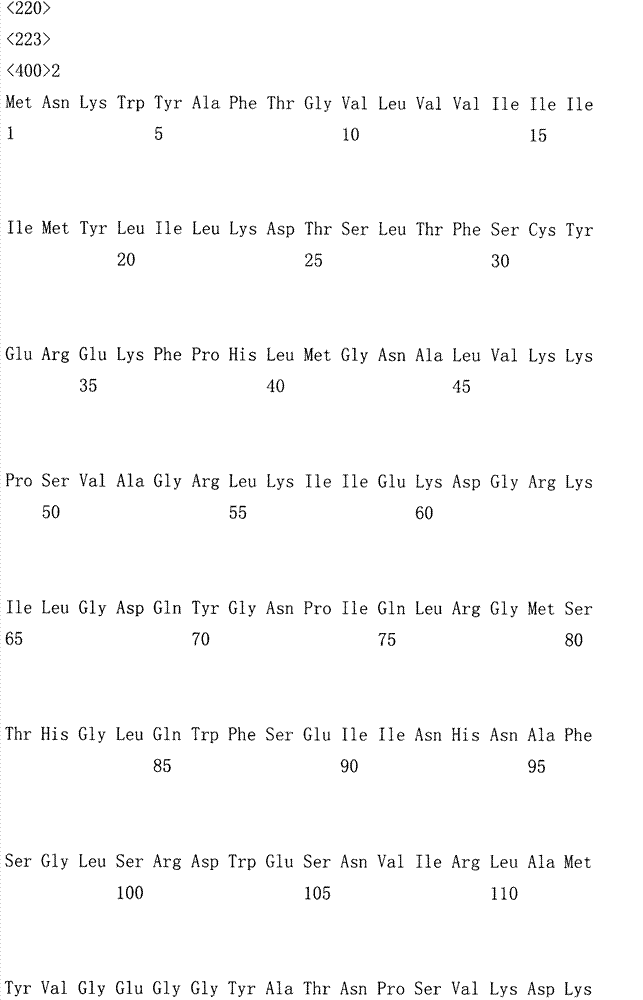Patents
Literature
66results about How to "Higher than vitality" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pullulan enzymatic mutant and preparation method thereof
ActiveCN102876650AHigher than vitalityHigh activityGlycosylasesVector-based foreign material introductionPullulanWild type
The invention discloses a pullulan enzymatic mutant with high specific activity and high thermal stability and a preparation method of the pullulan enzymatic mutant, and belongs to the field of genetic engineering and enzyme engineering. The invention improves the specific activity and the thermal stability of pullulan enzyme by site-specific mutagenesis, and provides a mutation scheme through which the catalytic specific activity and the thermal stability of the pullulan enzyme derived from debranch bacillus are improved. At least one property of the pullulan enzymatic mutant is changed: (1) the optimal reaction temperature is increased, (2), the thermal stability on the condition that the pH is 4.0 to 5.0 is improved; and (3) the specific activity on the condition that the pH is 4.0 to 5.0 is improved. The pullulan enzymatic mutants are more suitable for the production process of starch saccharification as compared with wild type pullulan enzyme.
Owner:山东黄三角生物技术产业研究院有限公司
Pullulanase mutant and preparation method thereof
ActiveCN103484443AHigher than vitalityHigh activityMicroorganism based processesFermentationArginineWild type
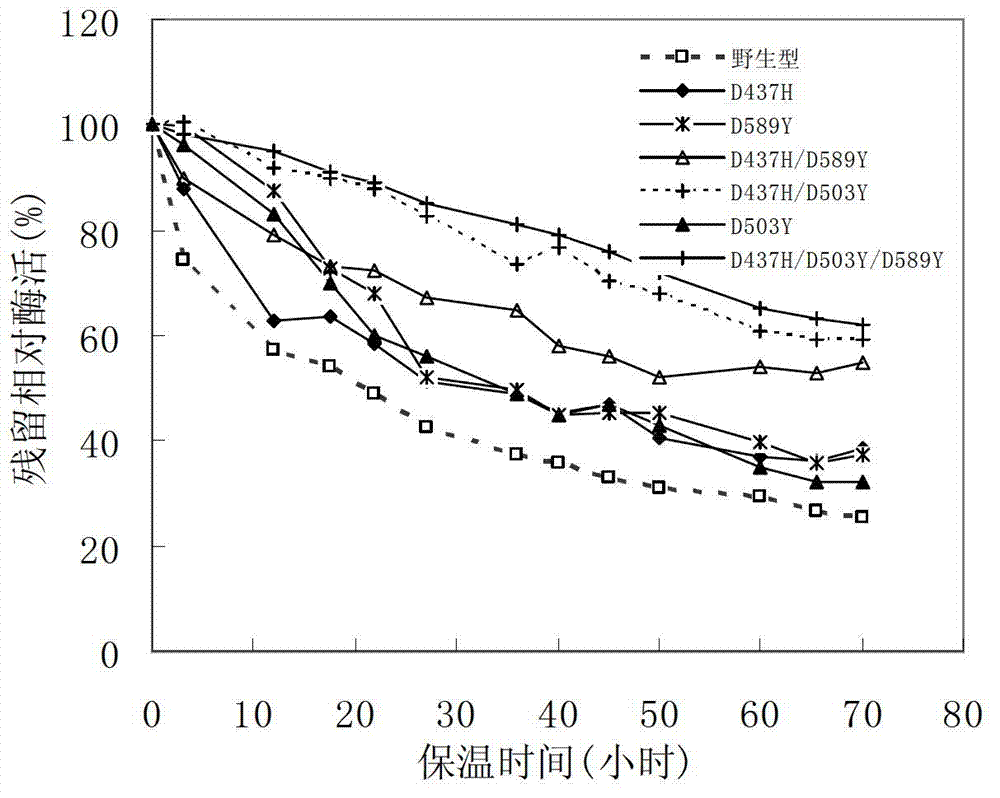
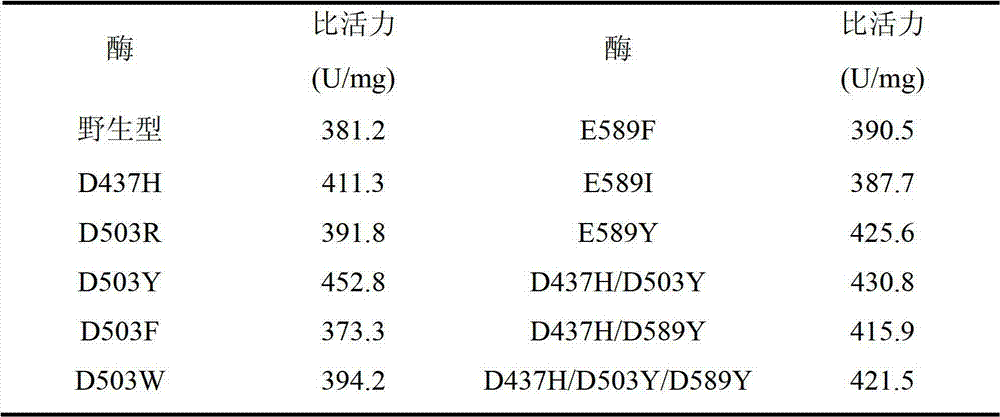
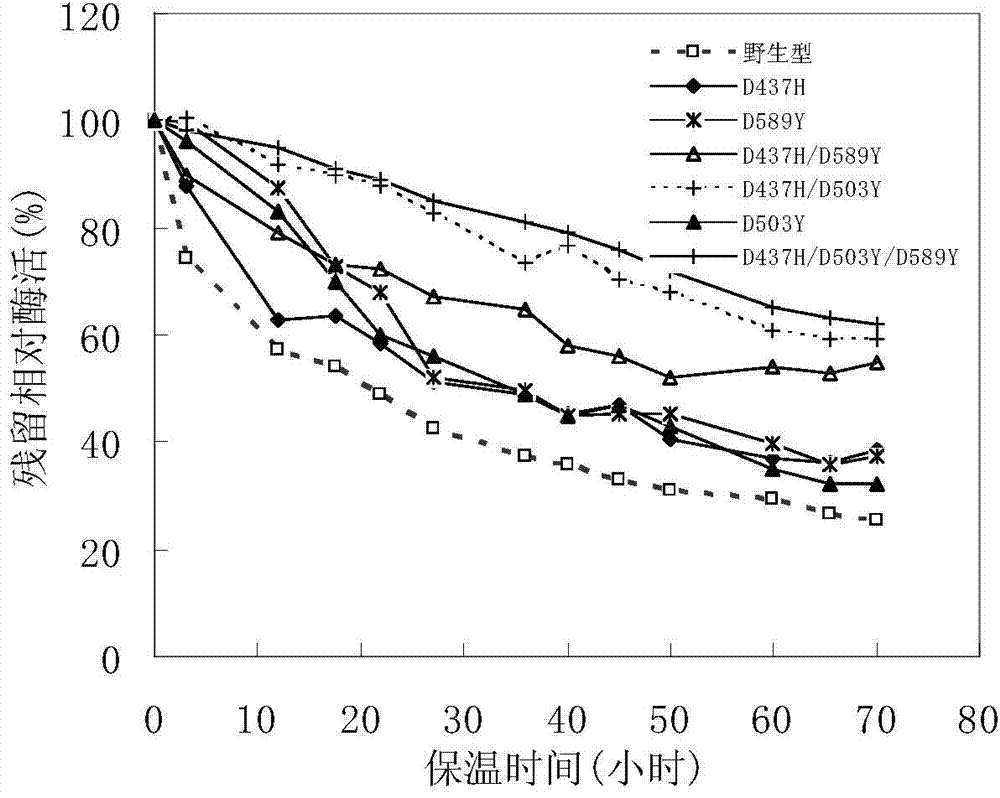
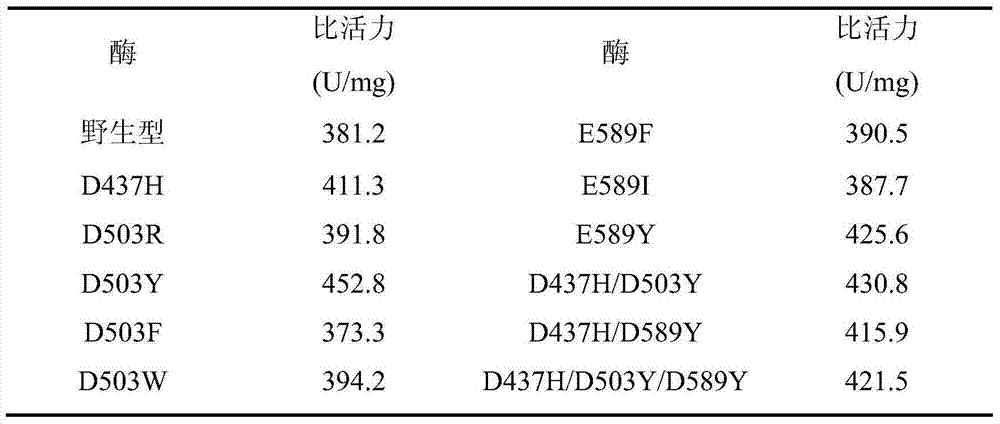
The invention discloses a pullulanase mutant having a high specific activity and a high thermal stability, and a preparation method thereof. The mutant comprises one, two or three substituents relative to debranching Bacillus spp Pullulanase active amino acid residues and related with the thermal stability of pullulanase; and the active amino acid residues comprise 503th aspartic acid, and can be mutated to form arginine, phenylalanine and tryptophan or tyrosine, the above mutation improves the high specific activity and the high thermal stability of the pullulanase, and at least one of the cases comprising the rise of a best reaction temperature, the improvement of the thermal stability at a pH value of 4.0-5.0 and the improvement of the specific activity at a pH value of 4.0-5.0 is realized. The mutant is more suitable for the starch saccharification production process than wild pullulanase.
Owner:JIANGNAN UNIV
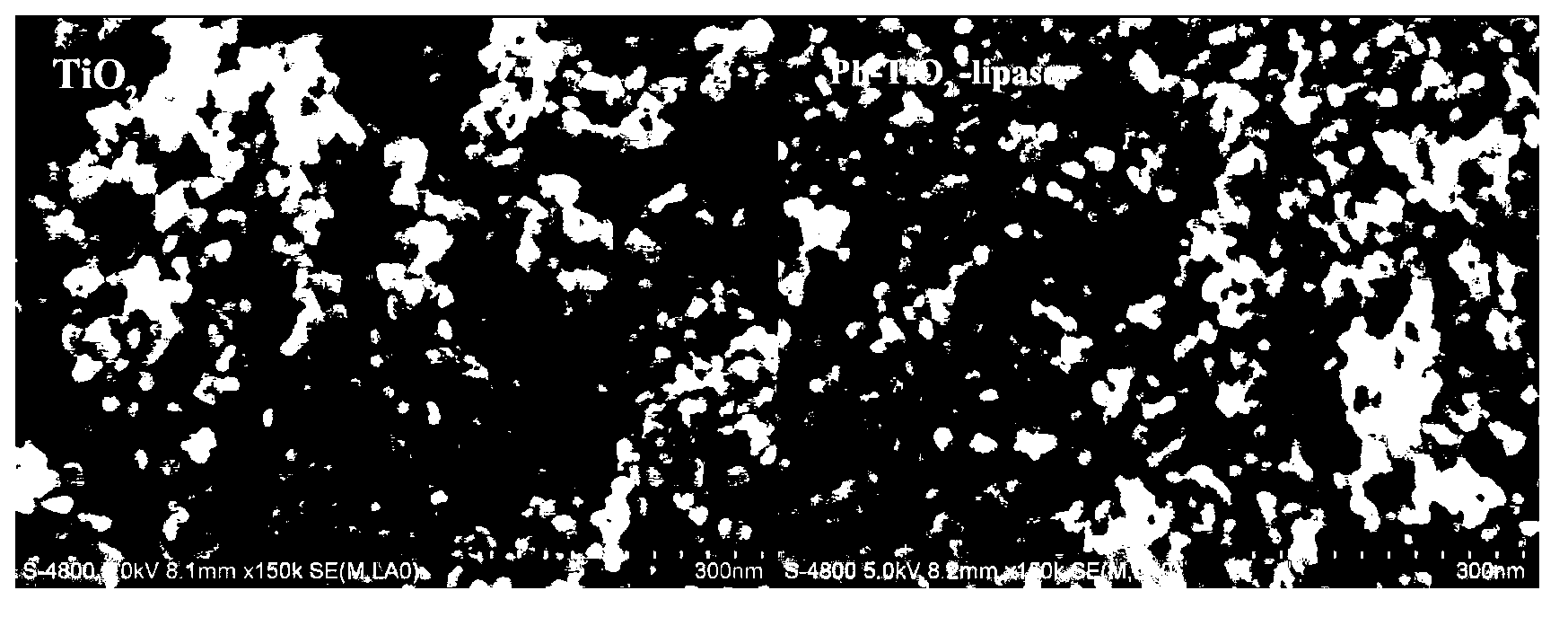
Immobilized lipase and preparation method and application thereof
InactiveCN103232992AHigh energy recovery rateSignificant activationHydrolasesChemical industryEnzymatic synthesisProtein molecules
The invention belongs to the fields of biochemical engineering and enzyme engineering, and relates to immobilized lipase and a preparation method and application thereof. The method comprises the steps of carrying out hydrophobic modification on surface of preprocessed mesoporous TiO2 by a silane coupling agent, so as to obtain a surface-modified mesoporous TiO2 carrier; taking modified mesoporous TiO2 as a carrier to carry out immobilized preparation of immobilized lipase on lipase; carrying out aromatic ester enzymatic synthesis and enzymatic enantioseparation of a drug intermediate by the immobilized lipase in an organic phase, and carrying out purification research on the lipase by the carrier. The carrier obtained by the preparation method can selectively absorb a lipase protein molecule from crude enzyme for lipase fermentation. Therefore, the method is simple in immobilization technology, mild in condition, less in enzyme activity loss in the immobilization process, and high in active recovery rate. The prepared mmobilized lipase has good stereoselectivity and catalytic activity in the organic phase, and has wide application prospect.
Owner:NANJING UNIV OF TECH
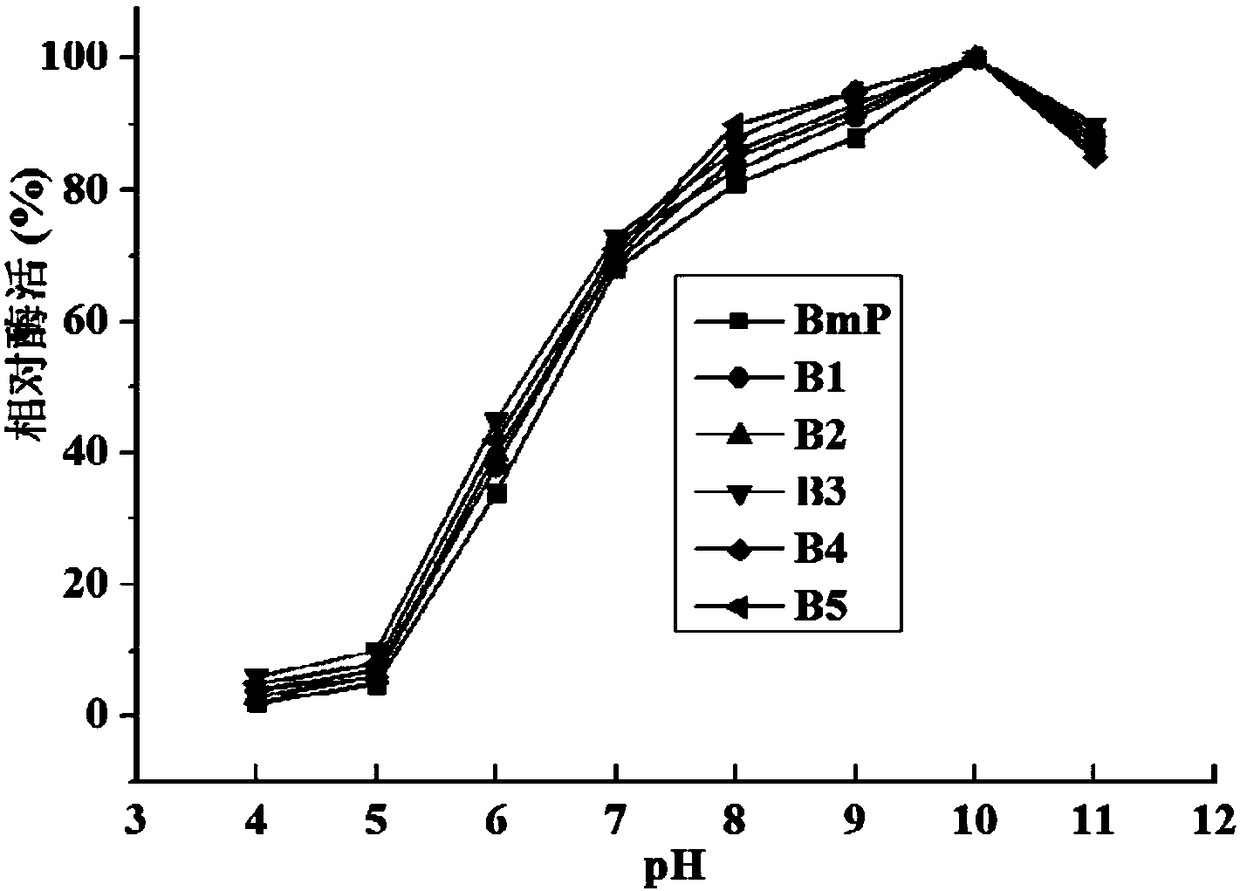
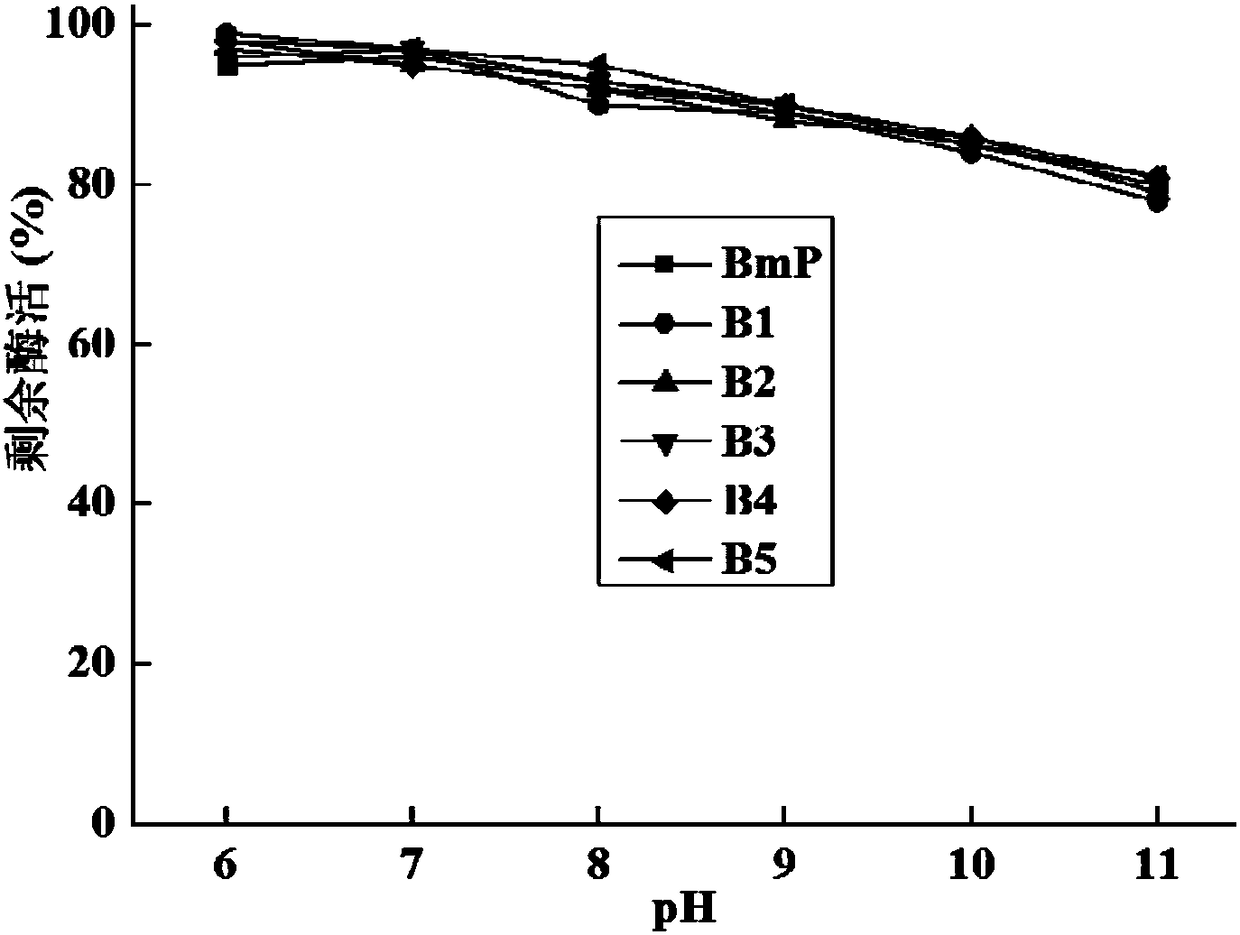
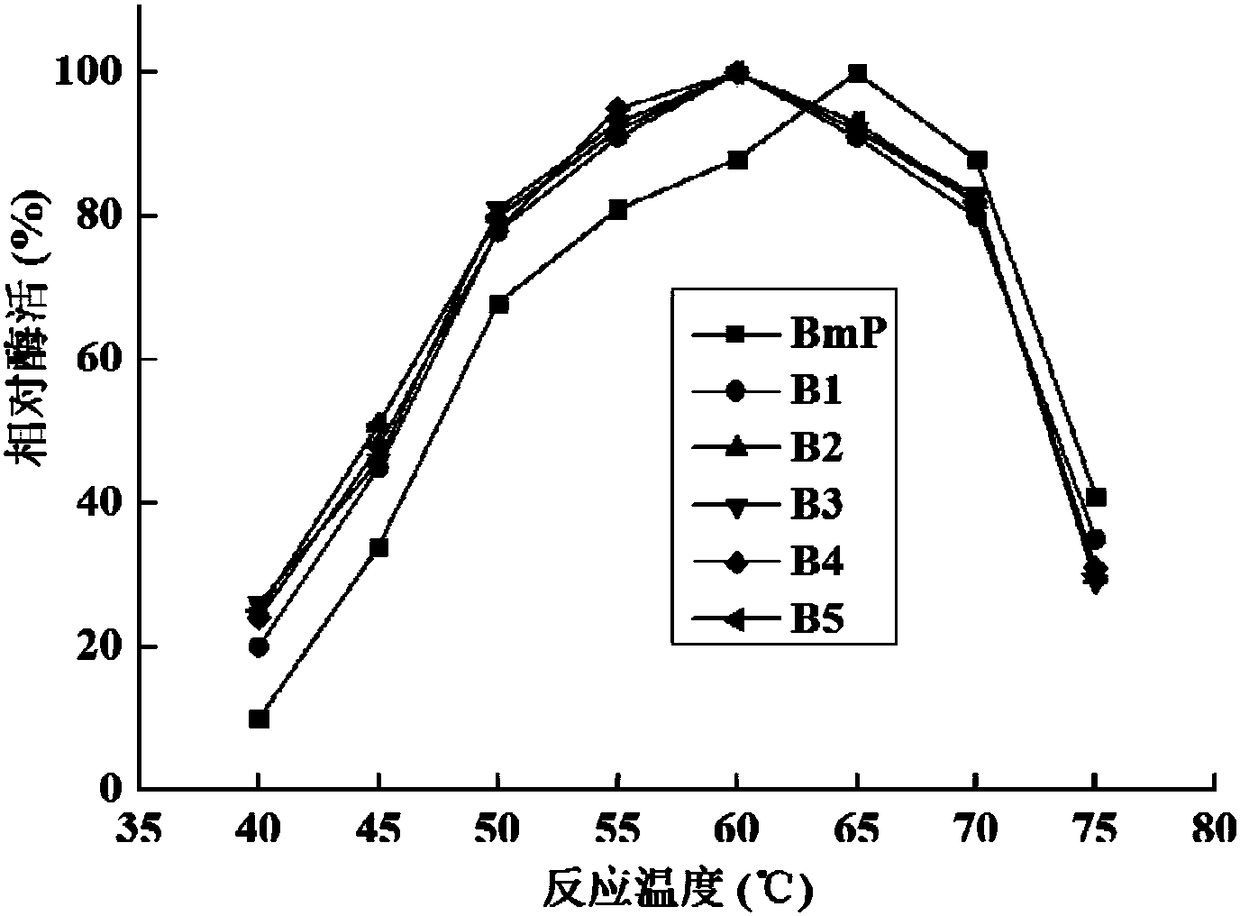
Alkaline protease BmP mutant for improving specific activity and coding gene thereof
ActiveCN108570461AHigher than vitalityReduce manufacturing costBacteriaHydrolasesAlkaline proteaseADAMTS Proteins
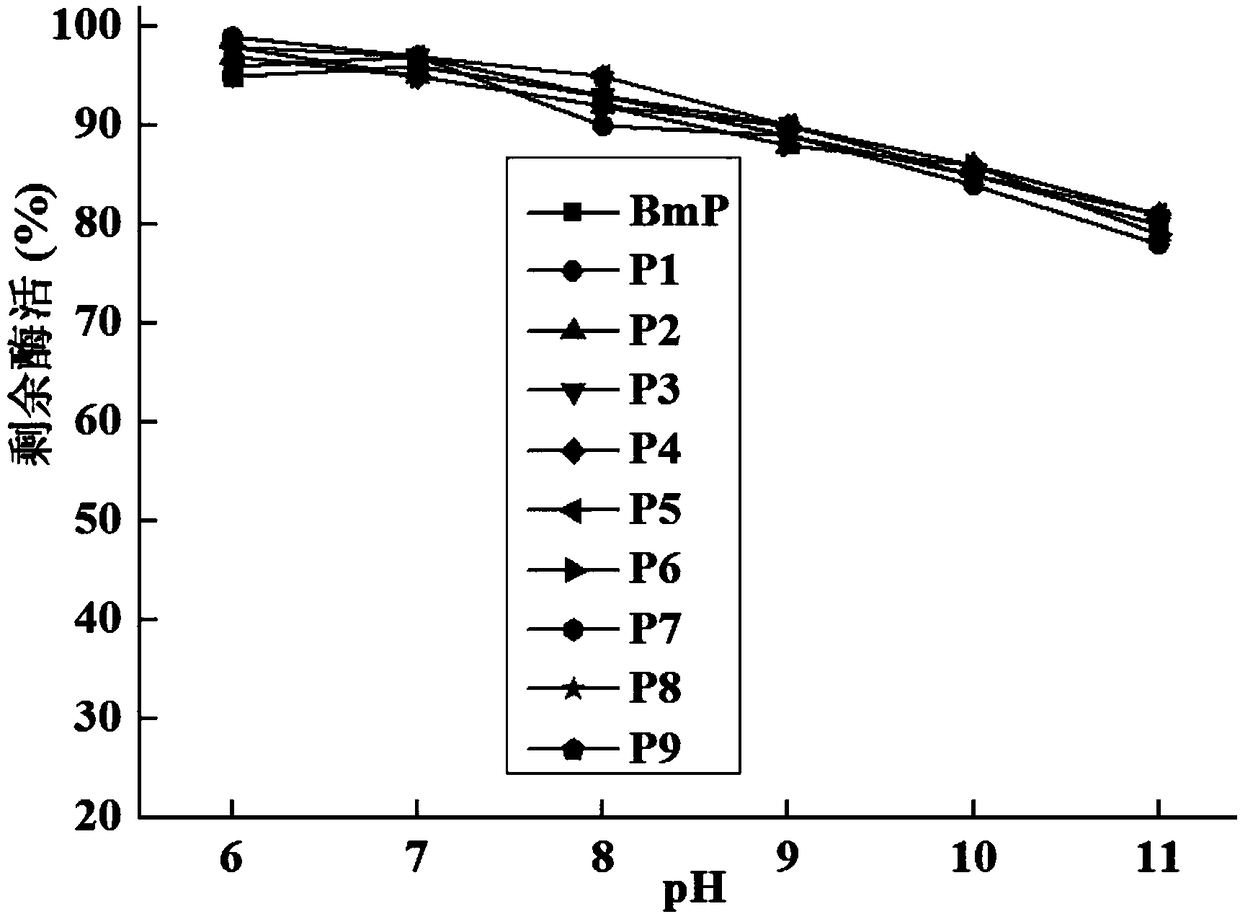
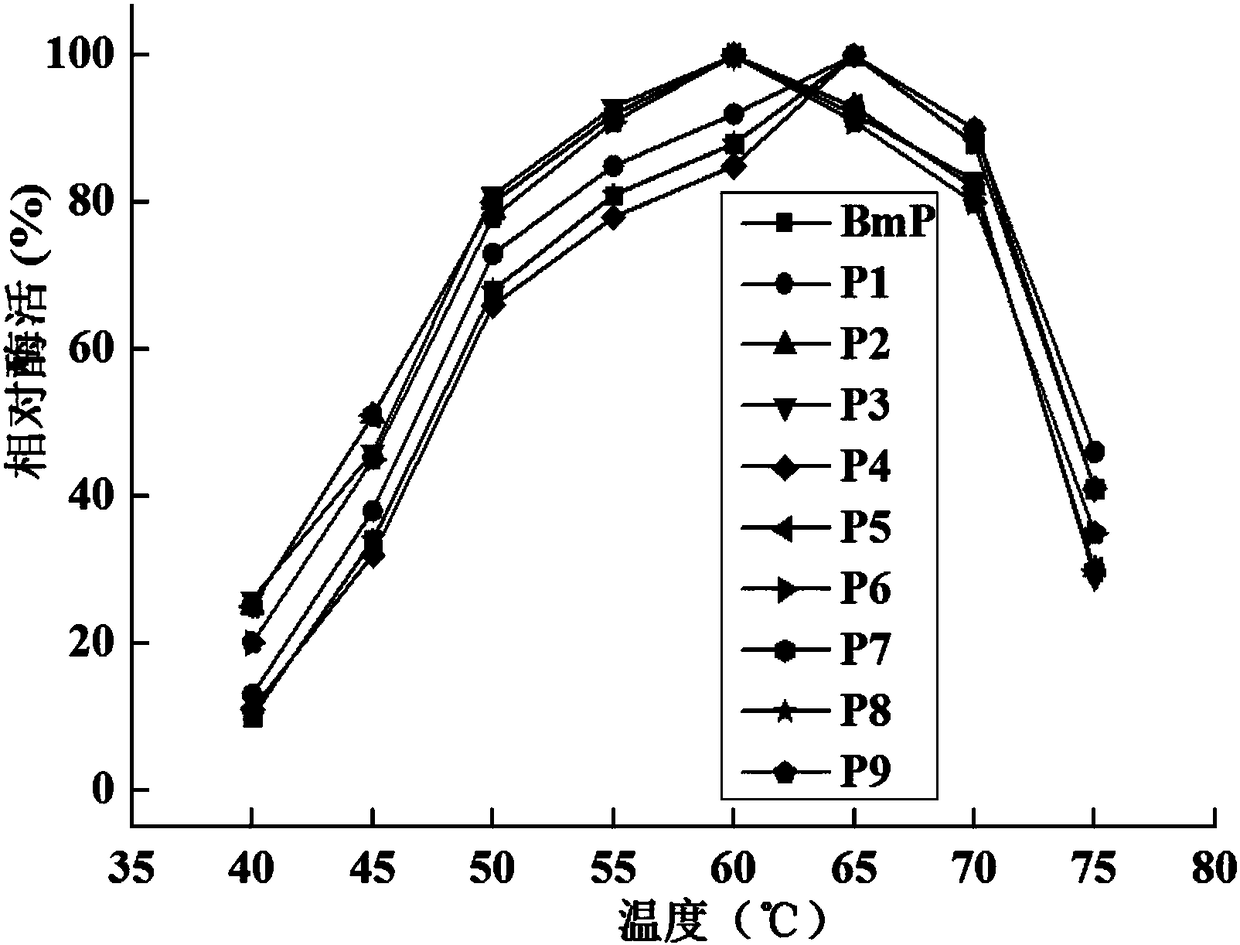
The invention relates to the field of protein molecular modification, in particular to an alkaline protease BmP mutant for improving specific activity and a coding gene thereof. The mutation site of the alkaline protease BmP mutant is as follows: the 236th site of the amino acid sequence shown as SEQ ID NO.11 of alkaline protease BmP is mutated from N to G or C or D, and the 293th site is mutatedfrom N to S or K or M. Experiments confirm that the mutant has higher specific activity than original enzyme, and the production cost of alkaline protease is reduced, so as to lay a foundation for theindustrial application of BmP.
Owner:横琴仲泰生物医药有限公司
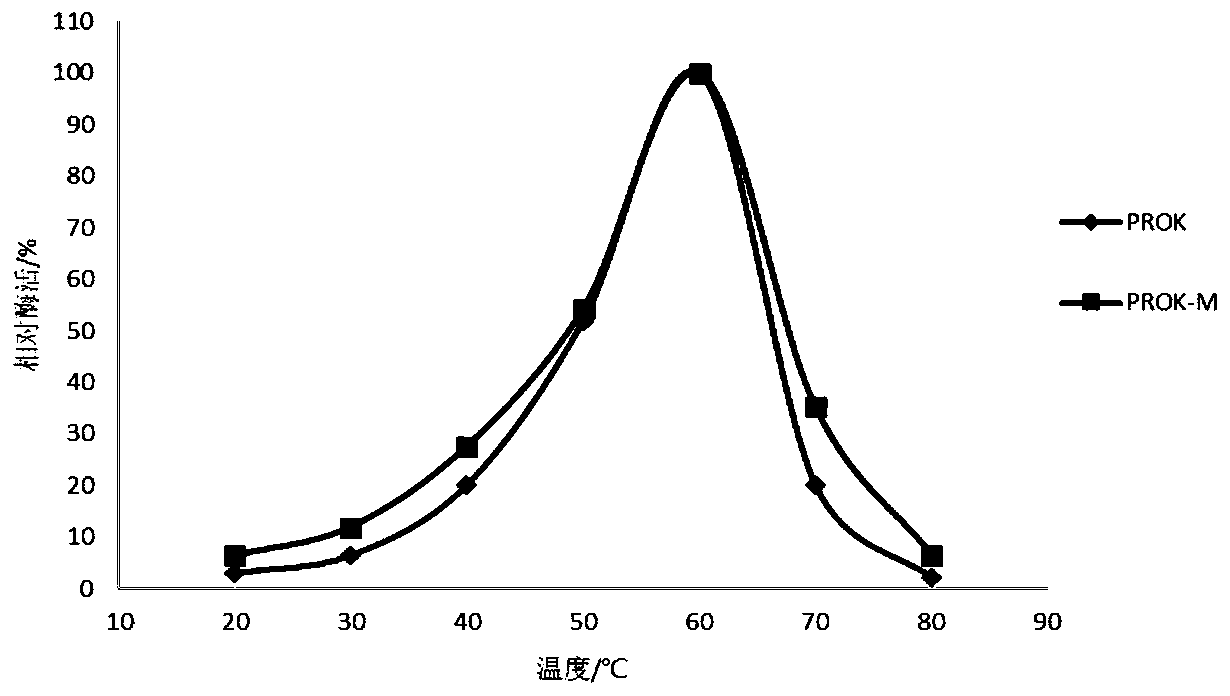
Alkaline protease mutant PROK-M with increased specific activity and thermal stability, and coding gene and applications thereof
ActiveCN109722428AHigher than vitalityImprove thermal stabilityFungiHydrolasesAlkaline proteaseWild type
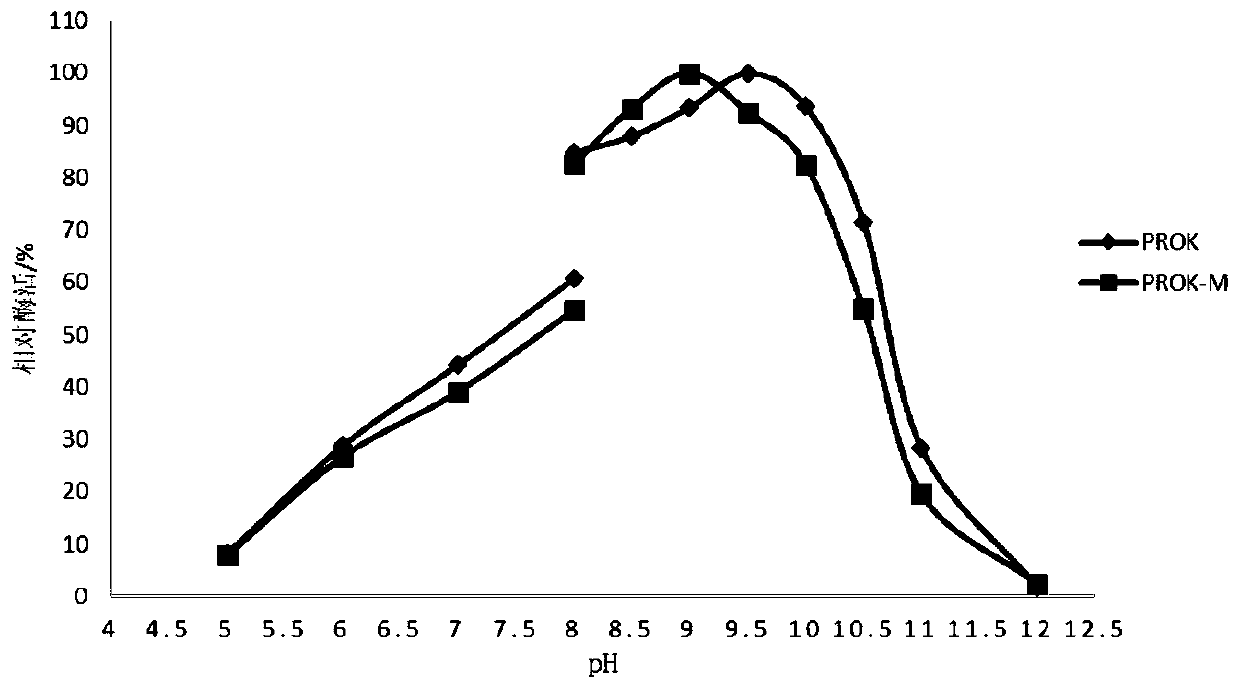
The invention belongs to the technical field of agricultural biology, and specifically relates to an alkaline protease mutant with increased specific activity and thermal stability, and a coding geneand applications thereof. The amino acid sequences of the alkaline protease mutant are shown as SEQ ID No.3. Compared with the specific activity of a wild type, the specific activity of the mutant canbe enhanced 15%; the optimum temperature of enzymatic reaction can be maintained unchanged; the optimum pH value can be reduced 0.5; and compared with the wild type, the thermostability of the mutantcan be enhanced under conditions of 55 DEG C and 60 DEG C. Thus, compared with the activity of the wild type, the alkaline protease mutant PROK-M can be higher in activity and more high temperature resistant.
Owner:INST OF ANIMAL SCI OF CHINESE ACAD OF AGRI SCI
Composite carrier for preparing immobilized protein, polypeptide or oligopeptide and preparation method and application thereof
ActiveCN104277111AEx situ and stable immobilizationLarge specific surface areaBiocideOther chemical processesImmobilized proteinOligopeptide
Provided are a composite carrier for immobilization of a protein, polypeptide or oligopeptide, a preparation method and an application thereof. The composite carrier is a porous material which comprises: (1) a porous organic foam material containing open pores; and (2) a crosslinked product having aldehyde groups and immobilized on the surface of the walls of one or more pores of the porous organic foam material, wherein the aldehyde groups are able to react with the protein, polypeptide or oligopeptide, and the crosslinked product are formed by chitosan via a crosslinking reaction with a polyaldehyde compound.
Owner:BIORIGHT WORLDWIDE
Phosphotriesterase mutant as well as preparation method and application thereof
InactiveCN102796714AImprove hydrolysis activityHigh catalytic efficiencyBacteriaHydrolasesOrganophosphate poisonWild type
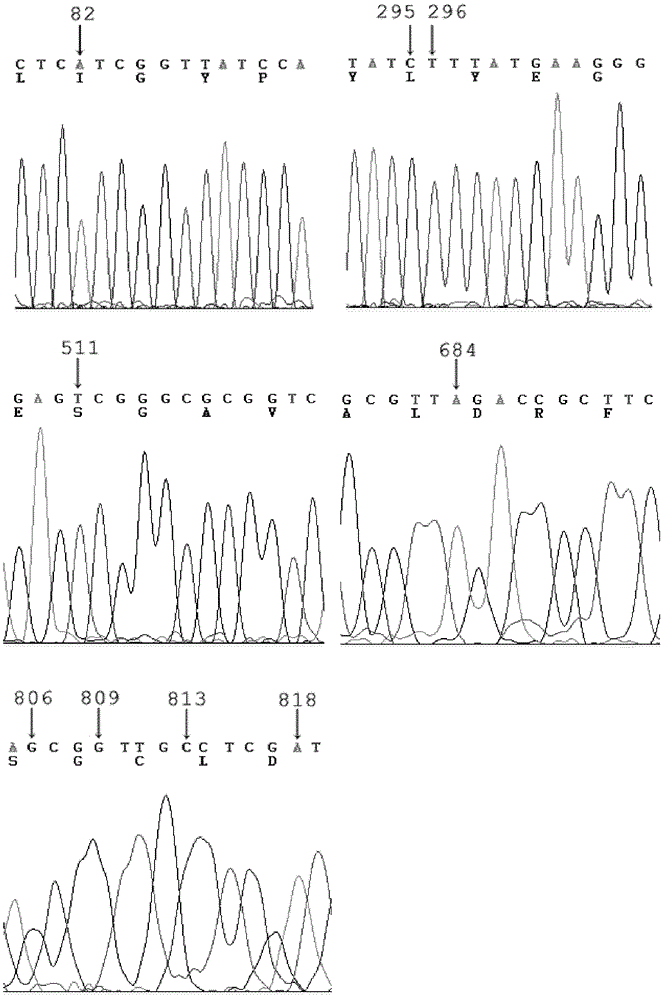
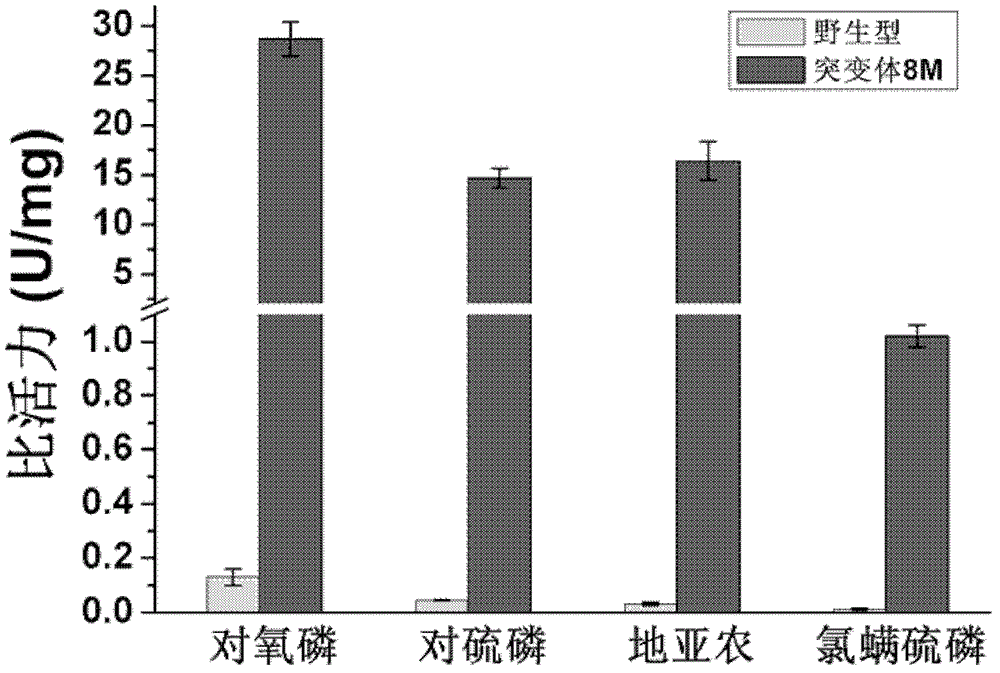
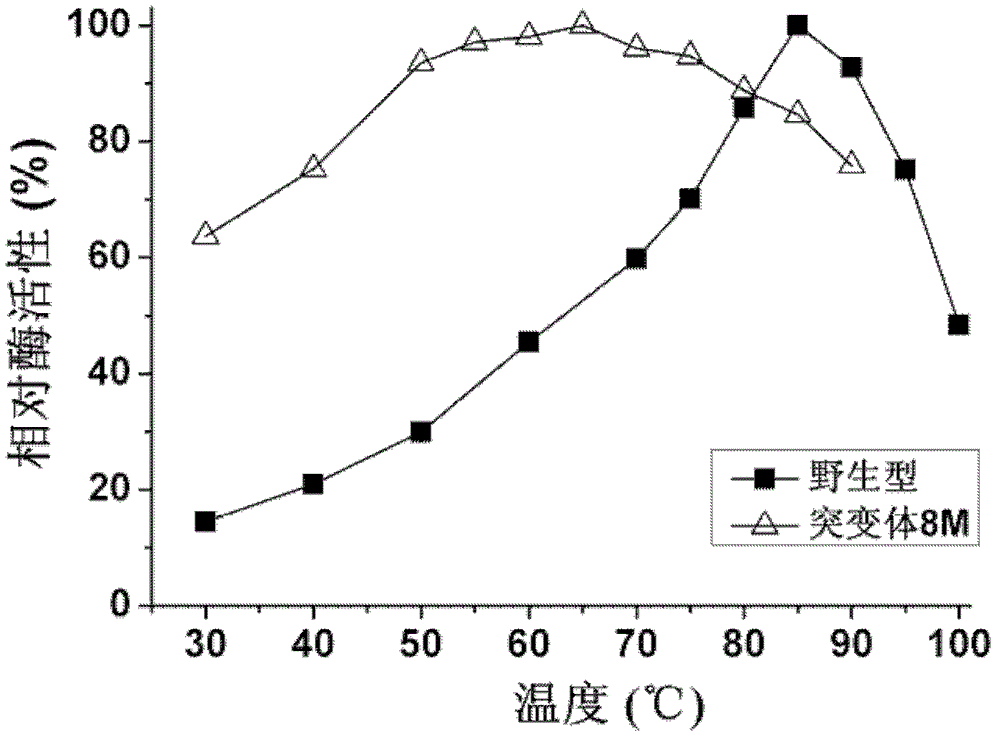
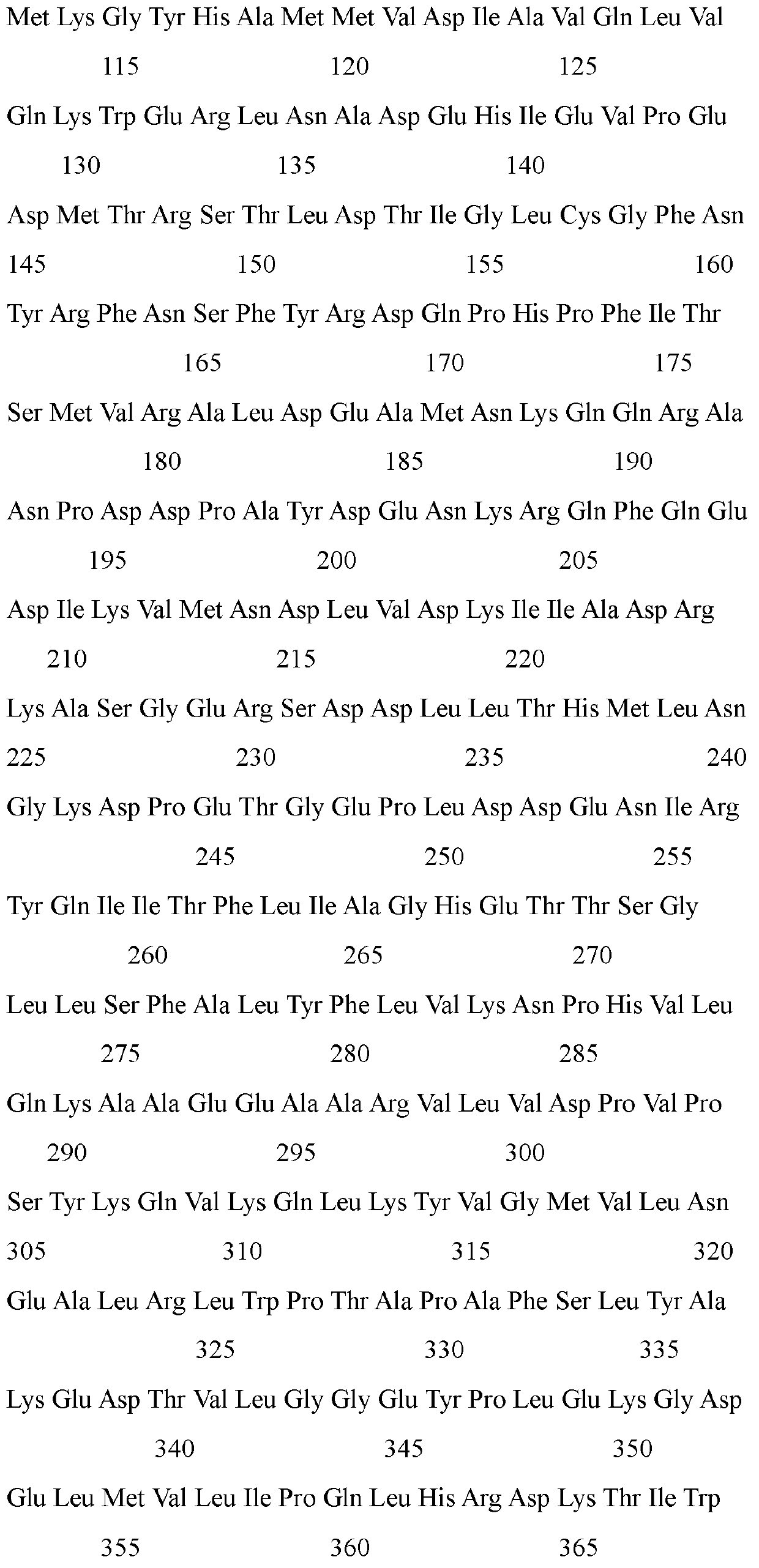
The invention provides a phosphotriesterase mutant as well as a preparation method and application of the phosphotriesterase mutant. According to the invention, a mutant with phosphotriesterase activity markedly improved is finally obtained by starting from a phosphotriesterase gene which is from Geobacillus kaustophilus HTA426 of thermophilic bacteria and then carrying out rounds of mutation and screening through an error-prone PCR (Polymerase Chain Reaction) method; and the mutations involved in amino acid sequence of the mutant are Phe28Ile, Tyr99Leu, Thr171Ser, Phe228Leu, Asn269Ser, Val270Gly, Trp271Cys and Gly273Asp. Compared with common organic phosphorous insecticides, the wild type specific activity of the mutant is markedly improved and the mutant has wide application prospect in the field of biodegradation of organic phosphorous poisions.
Owner:SHANGHAI JIAO TONG UNIV
Cytochrome P450 BM-3 (L148S/Q 229R) variant enzyme and coding gene and use thereof
InactiveCN102747052AImprove thermal stabilityHigh catalytic activityBacteriaMicroorganism based processesAnserineArginine
The invention discloses a cytochrome P450 BM-3 (L148S / Q 229R) variant enzyme and a coding gene and use of the cytochrome P450 BM-3 (L148S / Q 229R) variant enzyme. The amino acid sequence of the variant enzyme is shown as the SEQ ID NO. 1. Two mutational sites are added on the basis of the cytochrome P450 BM-3 (F87V / A74G / L188Q) variant enzyme, i.e., the No. 148 leucine (L) is mutated into serine (S), and the No. 229 glutamine (Q) is mutated into arginine (R). Compared with a male parent, the variant enzyme disclosed by the invention is higher in affinity to substances and thermal stability.
Owner:NINGBO INST OF TECH ZHEJIANG UNIV ZHEJIANG
Endoglucanase mutant, gene, engineering bacterium and application thereof
ActiveCN111690629AHigher than vitalityImprove reaction catalytic efficiencyFungiMicroorganism based processesArginineCellulase
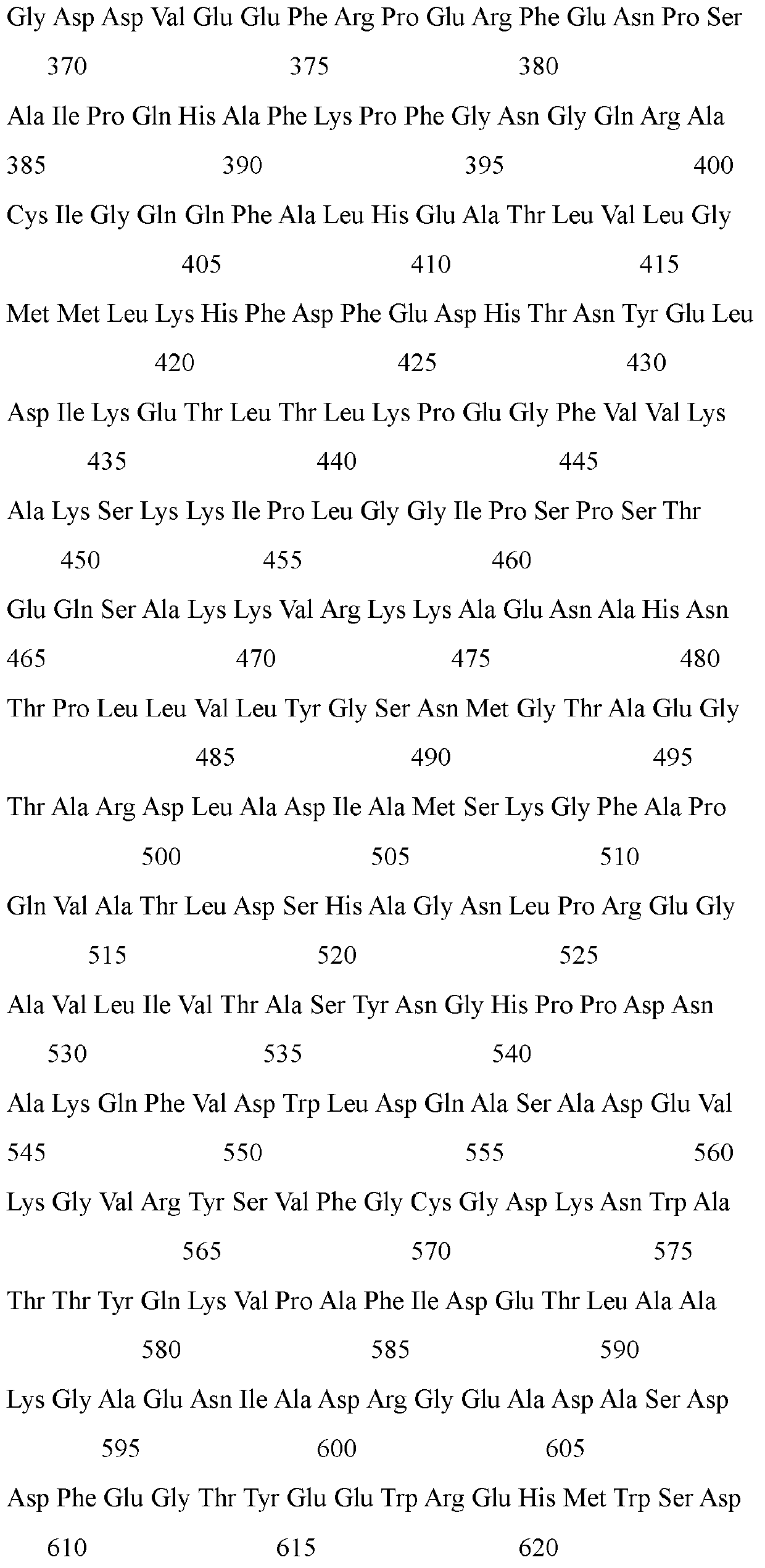
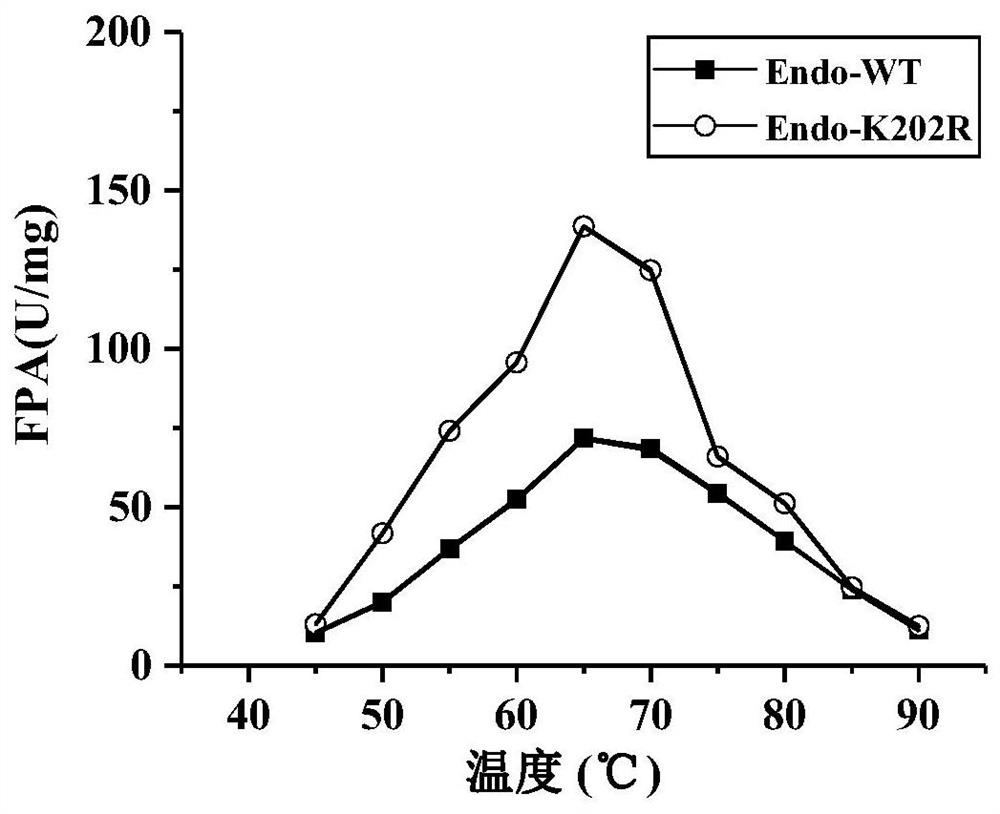
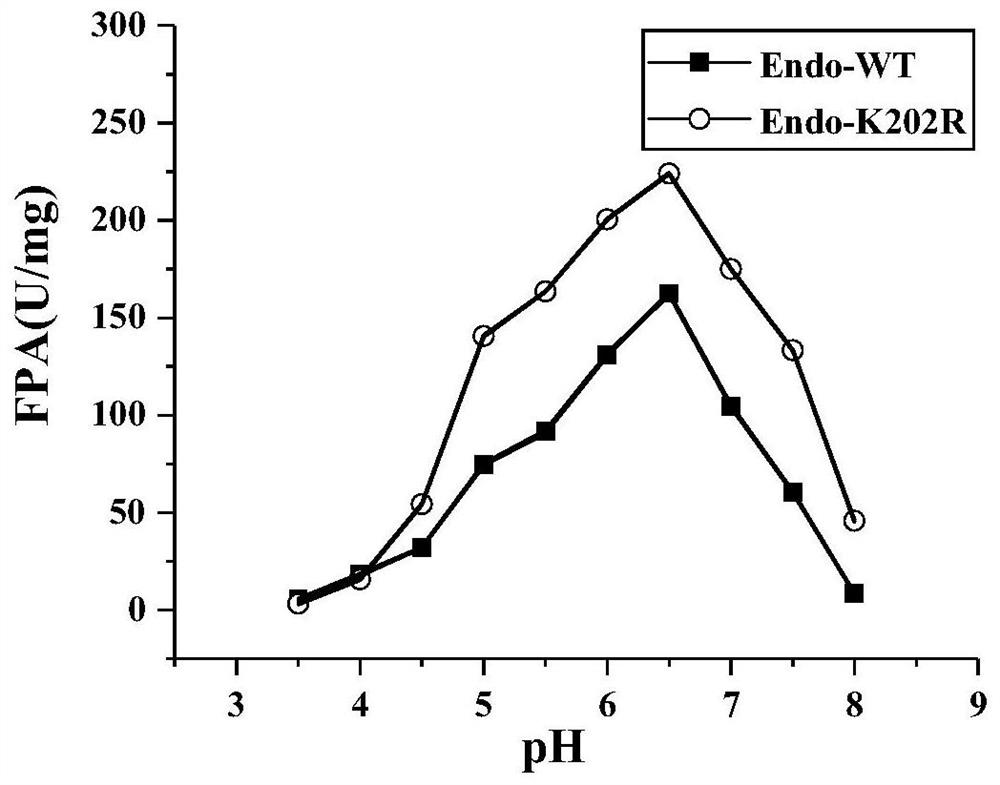
The invention discloses an endoglucanase mutant, a genetically engineered bacterium and application of the endoglucanase mutant in degradation of cellulose. The endoglucanase mutant is obtained by mutating lysine at the 202 <nd> position of an amino acid sequence shown in SEQ ID No. 3 into arginine. The specific activity of the endoglucanase mutant disclosed is obviously improved by about 1.4 times compared with that of a wild enzyme. When microcrystalline cellulose is used as a substrate; through the common reaction of cellulase and mutant enzyme, the reaction catalytic efficiency is remarkably improved by 23.5% compared with that of single degradation of a substrate with cellulase, it can be seen that the mutant enzyme remarkably improves the enzyme activity, the economic cost of industrial application can be reduced, and an application basis is provided for the mutant enzyme in the field of industrial production of food bioethanol and the like.
Owner:ZHEJIANG UNIV OF TECH +1
Low-temperature beta-xylosidase mutant with improved thermal stability and specific activity and encoding gene and application thereof
ActiveCN110699339AImprove thermal stabilityImprove stabilityFungiMicroorganism based processesBiotechnologyPapermaking
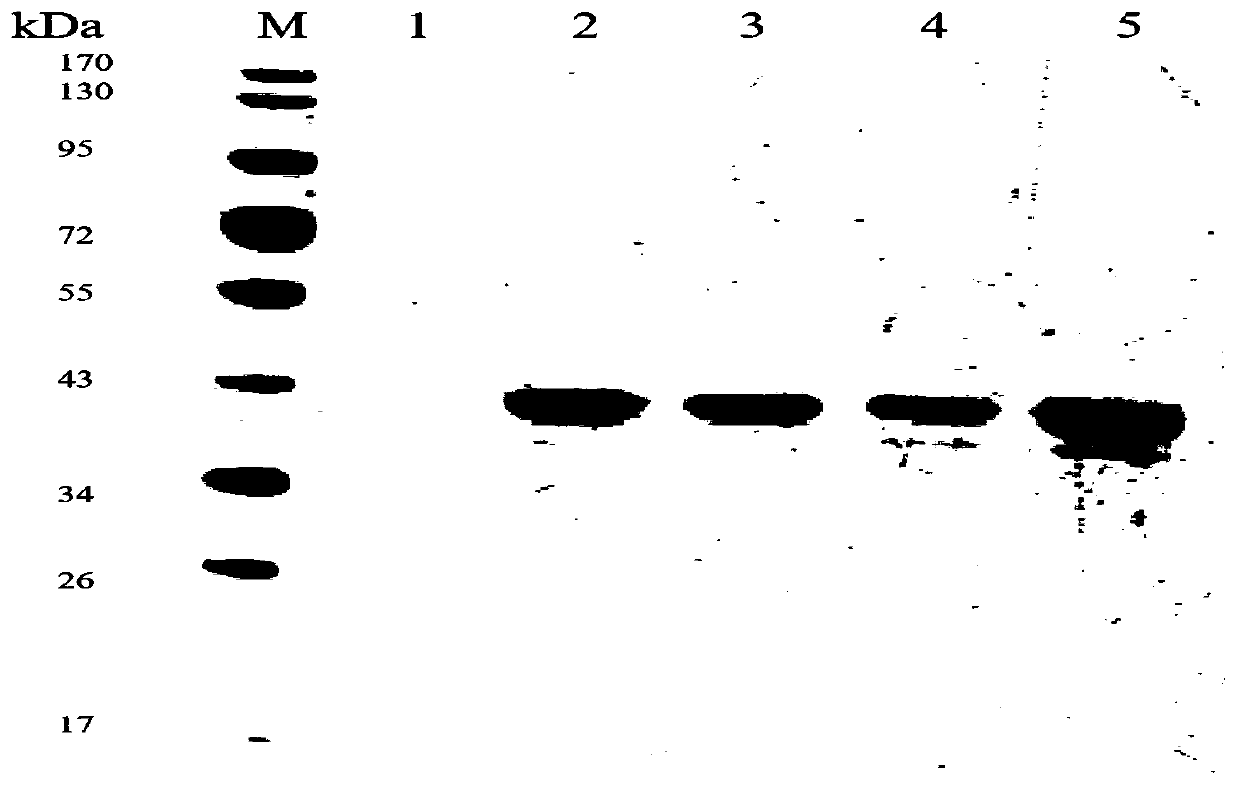
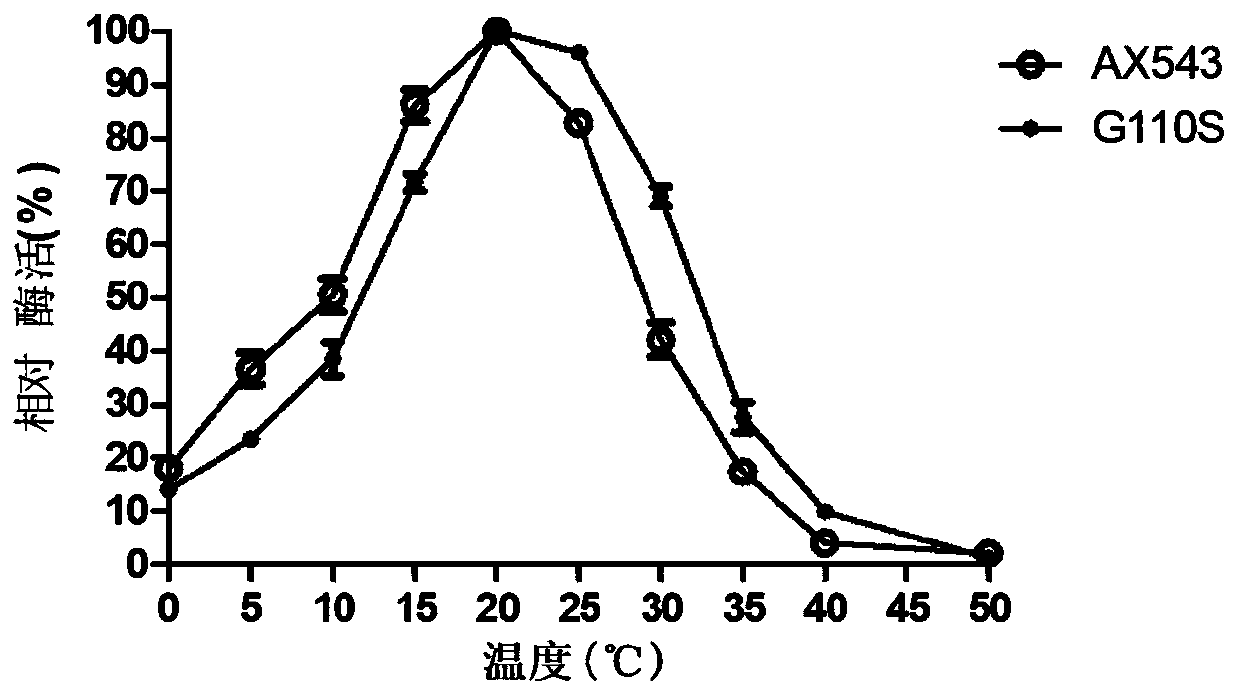
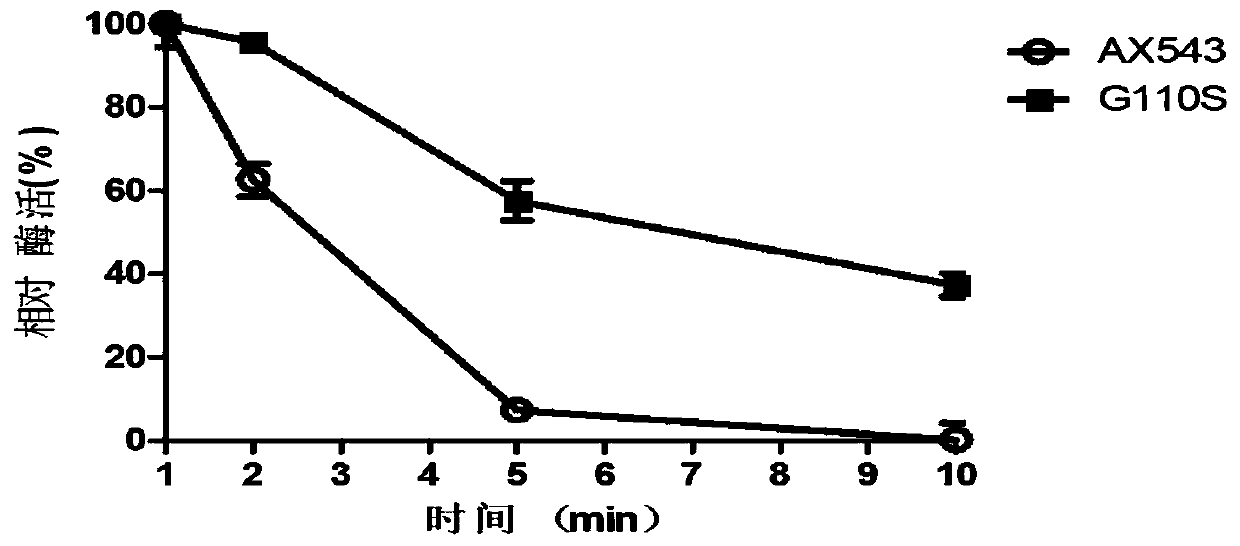
The invention relates to a low-temperature beta-xylosidase mutant with improved thermal stability and specific activity. The mutant is a low-temperature beta-xylosidase mutant G110S, and an amino acidsequence of the mutant is SEQ ID NO.1; alternatively, the mutant is a low-temperature beta-xylosidase mutant Q201R, and an amino acid sequence of the mutant is SEQ ID NO.2; and alternatively, the mutant is a low-temperature beta-xylosidase mutant loop2, and an amino acid sequence of the mutant is SEQ ID NO.3. The low-temperature beta-xylosidase mutant with improved thermal stability and specificactivity is more suitable for industrial applications, and has wider application prospects in the fields of food, feed, papermaking and the like, so that the application potential of low-temperature beta-xylosidase is expanded in food, pharmaceutical, papermaking, feed and other fields.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Method for improving mannase activity of bifunctional cellulase, cellulase mutant RMX-M and application
ActiveCN111676209ALive loss is smallHigher than vitalityFungiMicroorganism based processesGlycanCellulase
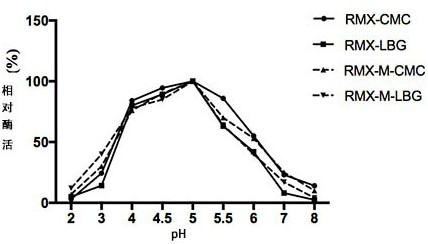
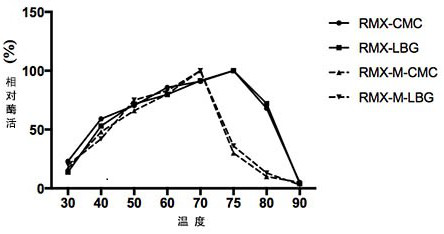
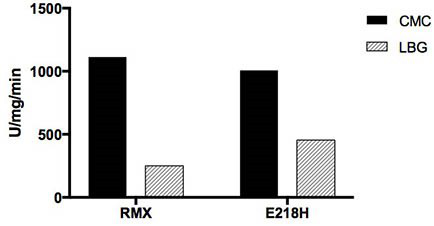
The invention relates to the technical field of agricultural biology, in particular to a method for improving the mannase activity of bifunctional cellulase, a cellulase mutant RMX-M and an application. An E218H mutant is obtained by implementing site-specific mutagenesis on an E218 site of wild cellulase with an amino acid sequence as shown in SEQ ID NO:1. Results show that the optimum pH value of an enzymatic reaction is not changed and the optimum temperature is reduced by 5 DEG C when two substrates, namely sodium carboxymethylcellulose and carob bean gum, are used for determination; whenthe carob bean gum is used as the substrate, the mannan specific activity of the mutant is improved by about 80% compared with that of the wild cellulase; and when the sodium carboxymethylcellulose isused as the substrate, compared with the cellulose specific activity of wild RMX, the specific activity of the mutant RMX-M is slightly reduced, and the capability of degrading the hemicellulose substrate mannan is improved on the basis of relatively small cellulase activity loss.
Owner:INST OF ANIMAL SCI OF CHINESE ACAD OF AGRI SCI
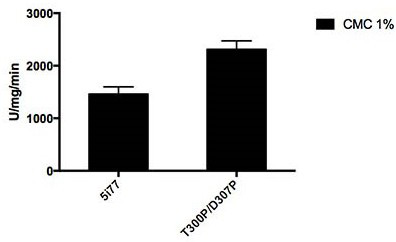
Method for improving cellulase activity, cellulase mutant 5I77-M and application
ActiveCN111676210AHigh catalytic activityHigher than vitalityFungiMicroorganism based processesBiotechnologySite-directed mutagenesis
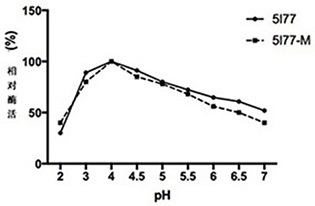
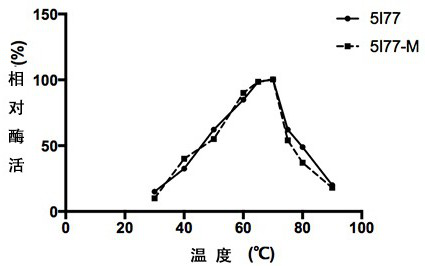
The invention relates to the technical field of agricultural biology, in particular to a method for improving the cellulase activity, a cellulase mutant 5I77-M and an application. A T300P / D307P mutantis obtained by carrying out site-specific mutagenesis on a T300 / D307 site of wild cellulase with an amino acid sequence as shown in SEQ ID NO:1. Results show that compared with the wild cellulase, the cellulase mutant has the advantages that the optimum pH value and the optimum temperature of the mutant do not change, and when sodium carboxymethylcellulose is used as a substrate, the specific activity of the mutant is improved by about 60% compared with that of the wild cellulase.
Owner:INST OF ANIMAL SCI OF CHINESE ACAD OF AGRI SCI
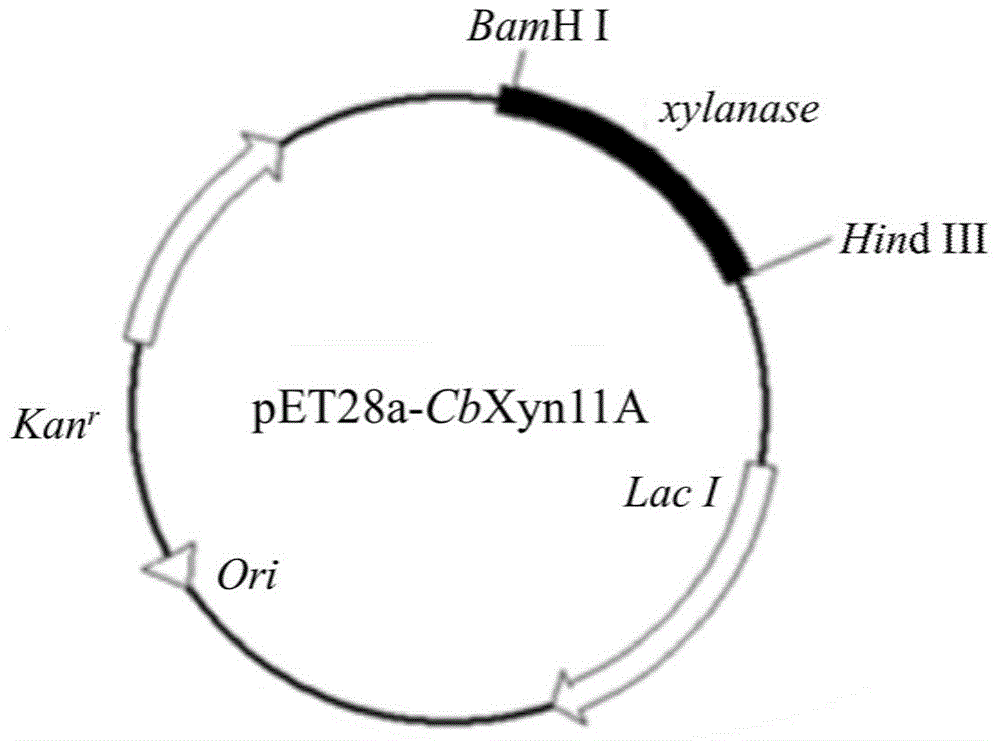
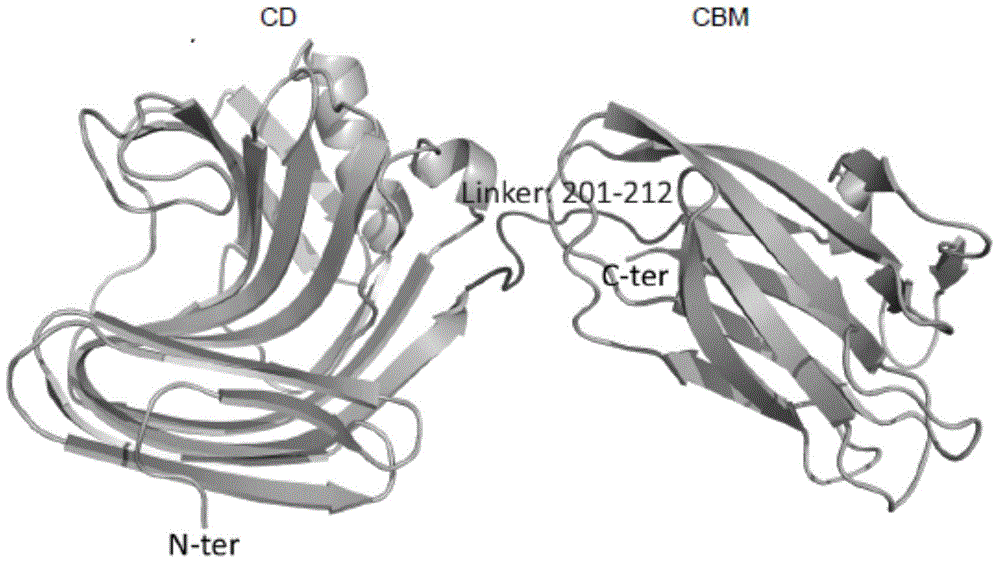
Genetic engineering xylanase and preparation and application of genetic engineering xylanase
ActiveCN104313000AImprove thermal stabilityHigh activityFermentationGenetic engineeringHeterologousThermal stability
The invention discloses genetic engineering xylanase which is a mutant, wherein a signal peptide is truncated at the tail end N of xylanase CbX, and meanwhile, efficient expression of a xylanase gene in a heterologous host cell is achieved. According to the genetic engineering xylanase disclosed by the invention, xylanase is modified, a carbohydrate combined structural domain at the terminal C is truncated and a disulfide bond is introduced at the tail end C, so that the molecular weight of the constructed xylanase protein mutant is smaller, the thermal stability is improved, and the specific vitality is further greatly improved. Due to the adoption of the manner, the xylanase and the mutant of the xylanase are of more potential and are applied to industrial production.
Owner:SHANGHAI JIAO TONG UNIV
Alkaline protease mutant with improved specific activity and coding gene of alkaline protease mutant
ActiveCN108384771AHigher than vitalityReduce manufacturing costHydrolasesGenetic engineeringAlkaline proteaseMutant
The invention relates to the field of gene engineering, and in particular relates to an alkaline protease BmP mutant with the improved specific activity and a coding gene of the alkaline protease BmPmutant. The mutation site of the alkaline protease BmP mutant refers to that the 178th site of the alkaline protease BmP as shown in amino acid SEQ ID NO.7 mutates into D,M,G,Q or T from S. The obtained mutant has higher specific activity than the original enzyme.
Owner:珠海市双指环投资有限公司
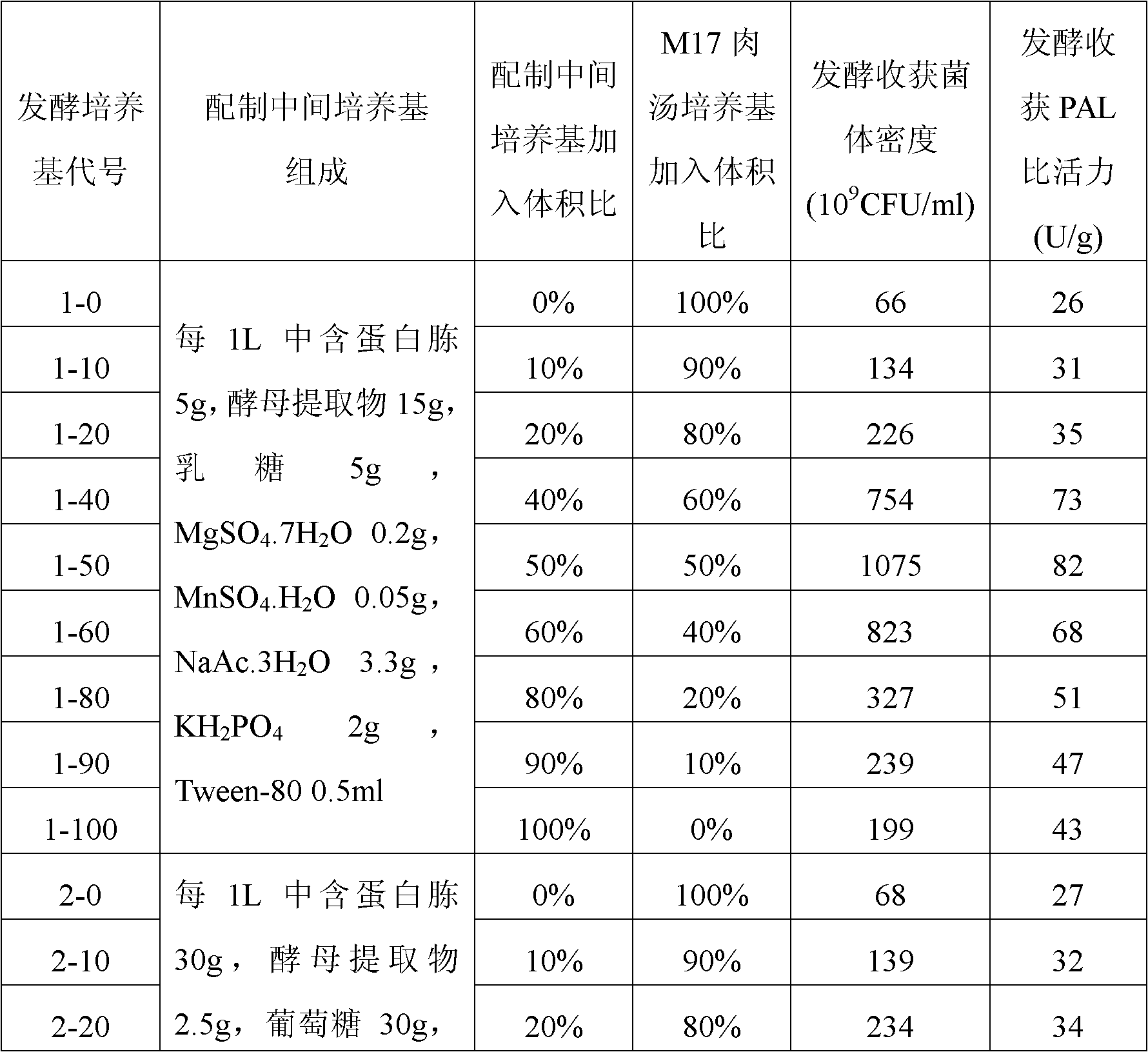
Preparation method of lactococcus lactis product for expressing phenylalanine ammonialyase
ActiveCN102358890AHigher than vitalityBacteriaMicroorganism based processesBiotechnologyStaphylococcus lactis
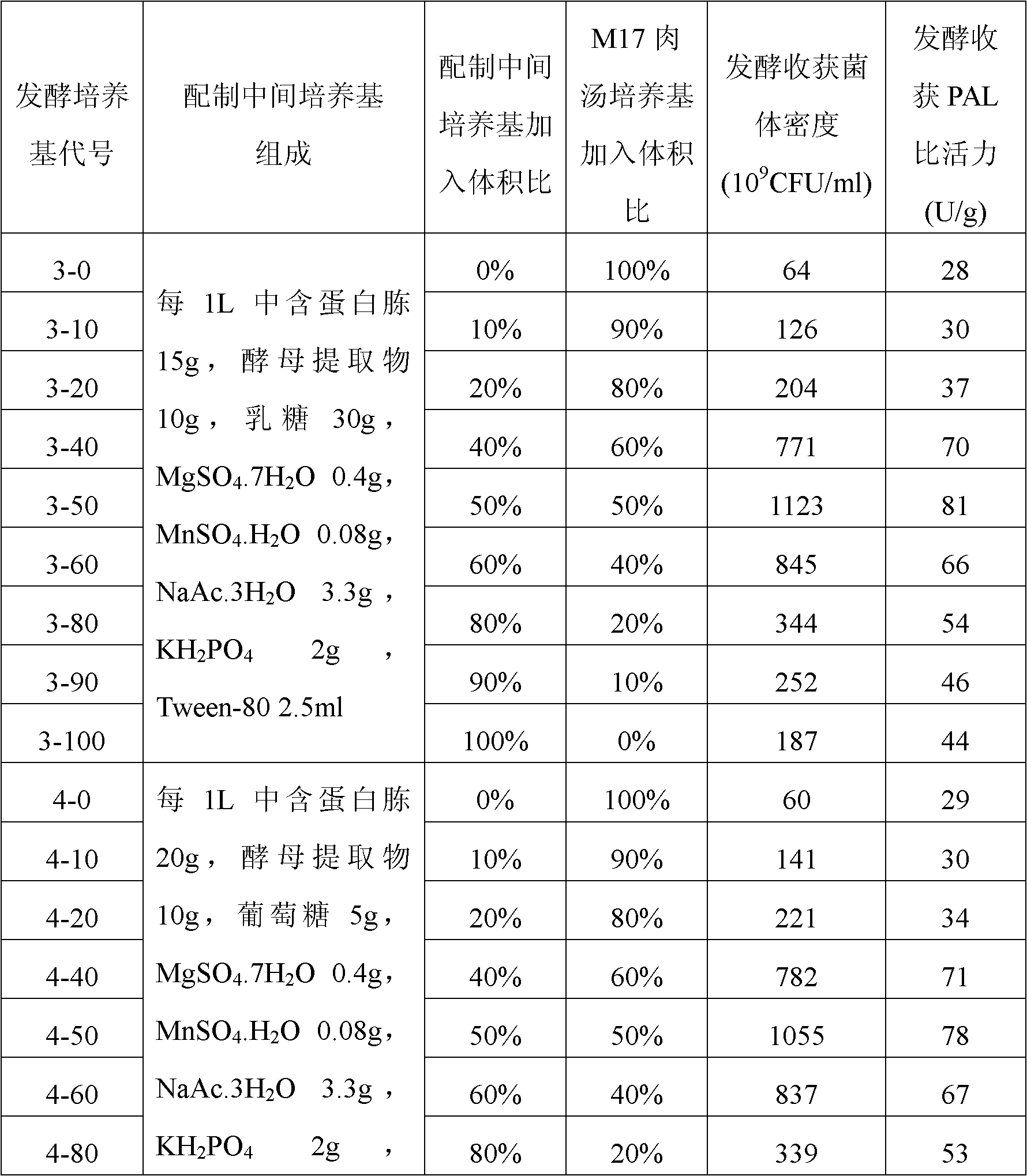
The invention belongs to the field of biomedicine, and relates to a preparation method of a lactococcus lactis product for expressing phenylalanine ammonialyase. The method sequentially comprises the following steps of: 1) acquiring genes for expressing the phenylalanine ammonialyase; 2) connecting the genes for expressing the phenylalanine ammonialyase and expression vectors suitable for transforming lactococcus lactis to form recombinant expression vectors; 3) transforming the recombinant expression vectors into competent lactococcus lactis; 4) screening lactococcus lactis strains for obtaining efficiently expressed phenylalanine ammonialyase; 5) fermenting the lactococcus lactis strains, and inducing the expressed phenylalanine ammonialyase; and 6) drying the strains obtained by fermentation, wherein the culture medium used in the fermentation of the step 5) is obtained by mixing an intermediate culture medium and an M17 broth culture medium in a volume ratio of 4:6-6:4; and regulating the pH value to be between 6.5 and 7.5. By adopting the technical scheme, the viable count of the obtained lactococcus lactis strains and the specific activity of the phenylalanine ammonialyase are greatly improved, wherein the viable count can reach over 10<12>CFU / ml, and the specific activity can reach over 80U / g.
Owner:BEIJING TRI PRIME GENE PHARMA CO LTD
Construction and application of free non-methanol induced pichia pastoris expression vector
InactiveCN110184291AWide variety of sourcesReduce manufacturing costFungiMicroorganism based processesPichia pastorisAgricultural science
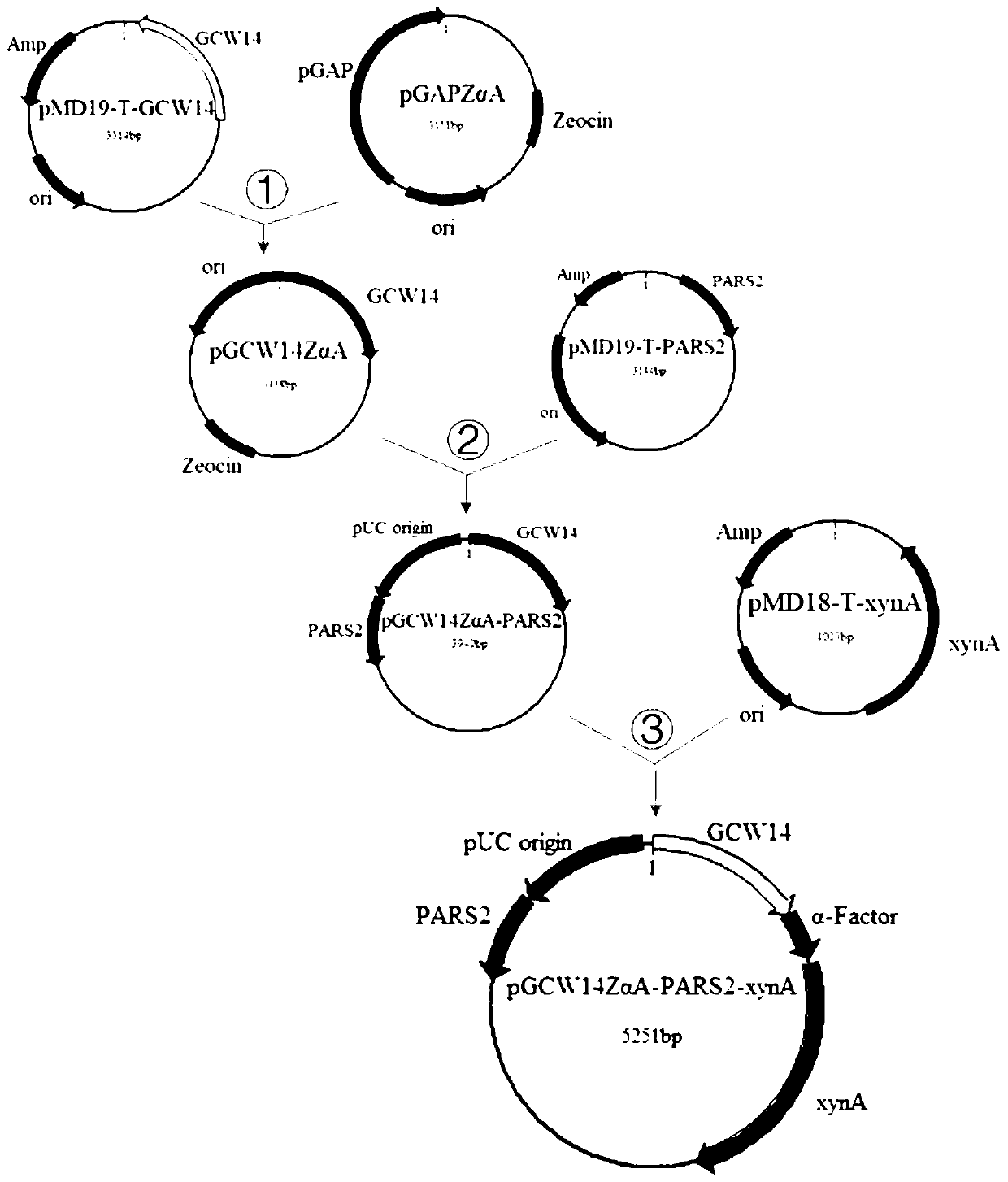
The invention relates to construction of a free non-methanol induced pichia pastoris expression vector and an application of the expression vector in enzyme recombination expression, and belongs to the technical field of genetic engineering. The non-methanol induced pichia pastoris expression vector is obtained by inserting a kluyveromyces lactis autonomous replication sequence PARS2 and a pichiapastoris promoter PGCW14 sequence into a vector framework, and constructing a free vector with a self-replication sequence. Compared with the general integrated recombinant pichia pastoris engineeringbacteria, the free recombinant pichia pastoris engineering bacteria constructed by the vector have obviously-improved capability of secreting and producing recombinant enzymes. In addition, the fermentation raw materials have wide sources, and the production cost is low.
Owner:JIANGNAN UNIV
Xylanase mutant with improved specific activity
ActiveCN112094834AReduce manufacturing costHigher than vitalityFungiFood processingWild typeMicrobiology
The invention aims to provide a xylanase mutant with improved specific activity and application of the xylanase mutant. The mutant comprises any one or more mutation sites in M78F, V143I, R148K, F163W, I177V and V206L. Compared with a wild type, the xylanase mutant has the advantages that the specific activity of the mutant is remarkably improved, so that the production cost of xylanase is reduced, and wide application of the xylanase in feed is promoted.
Owner:QINGDAO VLAND BIOTECH GRP
High-specific-activity acidic mannase mutant
ActiveCN111117987AHigh specific vitalityReduce manufacturing costFungiFood processingProtein proteinMolecular biology
The invention relates to the technical field of protein engineering modification, and particularly provides a high-specific-activity mannase mutant. Compared with a wild type, the mannase mutant provided by the invention has the advantages that the specific activity of the mannase mutant is generally improved by 4.4%-38.97%. The specific activity of the mannase mutant is improved, the production cost of mannase is reduced favorably, wide application of the mannase in the field of feed is accelerated, and the market prospect is wide.
Owner:山东康地恩生物科技有限公司 +1
Polyethyleneglycol modified aprotinin and preparation thereof
InactiveCN101412995AHigh specific vitalityLow immunogenicityPeptide/protein ingredientsAntipyreticDrug deliveryImmunogenicity
The invention discloses a polyethylene glycol-modified aprotinin and a preparation method thereof. The polyethylene glycol-modified aprotinin is characterized in that an amino group of aprotinin is connected with a chain of polyethylene glycol or a derivative thereof. The polyethylene glycol-modified aprotinin can improve the specific activity of the aprotinin, dramatically lowers the immunogenicity of the aprotinin and improves the stability of the aprotinin, thereby improving pharmacokinetic properties of the aprotinin and the tolerance, and reducing drug delivery times.
Owner:SHANGHAI INST OF PHARMA IND +1
Enzymology modification method for improving vitality of lipase
InactiveCN102242090AEnzymatic modification conditions are mildCause inactivationHydrolasesWater bathsImprovement rate
The invention provides an enzymology modification method for improving the vitality of a lipase. The method comprises the following steps: an enzyme solution with a certain concentration is prepared from trypsin and an HCl solution; a free lipase or an immobilized lipase and a protease which are added to a buffer solution with a certain pH value according to a certain proportion and well mixed are incubated for a period of time in a thermostatic water bath; the vitality of the lipase is determined by an olive oil emulsification process at a temperature of 37 DEG C; and the vitality improvement rate of a modified lipase is obtained through comparing with the activity of the lipase which is not processed by the protease. The method of the present invention has the advantages of simple step, easy operation, substantial improvement of the enzyme activity of the free lipase and the immobilized lipase (the vitality improvement rate of the free lipase reaches 32%, and the vitality improvement rate of the immobilized lipase reaches 38%), and mild reaction condition, and does not cause the denaturalization and the deactivation of the lipase because of extreme conditions.
Owner:SOUTH CHINA UNIV OF TECH
Nitrile hydratase lysine mutant HBA-K2H2R, coding gene and application
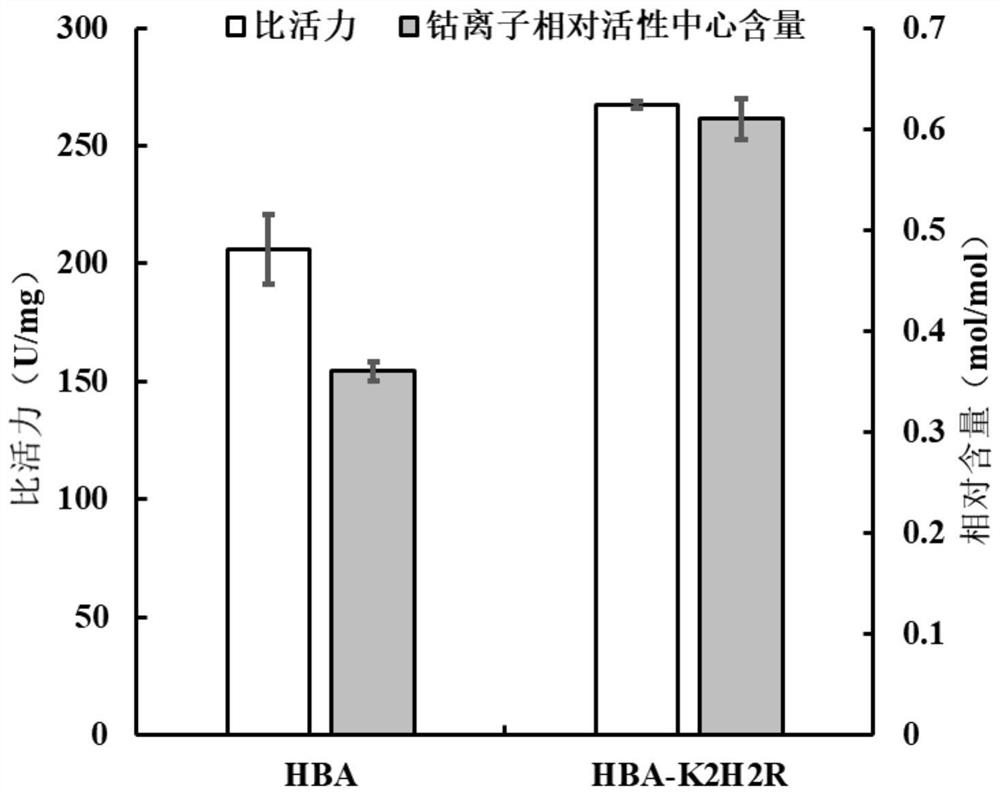
ActiveCN113151234AImproved efficiency of self-modificationHigher than vitalityBacteriaMicroorganism based processesChemical compoundMutant
The invention discloses a nitrile hydratase mutant, and particularly relates to a nitrile hydratase lysine mutant HBA-K2H2R, a coding gene of the nitrile hydratase lysine mutant HBA-K2H2R, a plasmid and a recombinant bacterium containing the coding gene of the nitrile hydratase lysine mutant HBA-K2H2R, and an application of the nitrile hydratase lysine mutant HBA-K2H2R in catalyzing an organic nitrile compound to prepare an amide compound. The amino acid sequence of the nitrile hydratase lysine mutant HBA-K2H2R is as shown in SEQ ID NO. 1, the most suitable pH value of the nitrile hydratase lysine mutant HBA-K2H2R is 9.0, and the most suitable temperature 35 DEG C. Compared with a wild enzyme HBA, the nitrile hydratase lysine mutant HBA-K2H2R has the improved alkaline activity, and can be applied to the biotechnical fields of production of acrylamide, nicotinamide and other amide compounds with high added values through biological catalysis, chemical fiber surface modification, alkaline sewage treatment and the like.
Owner:ZHEJIANG UNIV OF TECH
Phytase mutant with improved specific activity
The invention relates to the field of biotechnology, in particular to a phytase mutant, a preparation method and application thereof, a DNA molecule for encoding the phytase mutant, a carrier and a host cell. The mutant provided by the invention comprises a substitution of an amino acid at at least one position selected from the group consisting of 67, 72, 79, 115, 121, 295, 300, 307, 318, 329, 360, 376 and 385. The heat resistance of the mutant is remarkably improved, so that the phytase can be widely applied to feeds.
Owner:QINGDAO VLAND BIOTECH GRP
High-specific-enzyme-activity xylanase mutant and application thereof
ActiveCN112322604AHigh activityHigh affinityFood processingGenetic engineeringSpecific enzymeXylanase
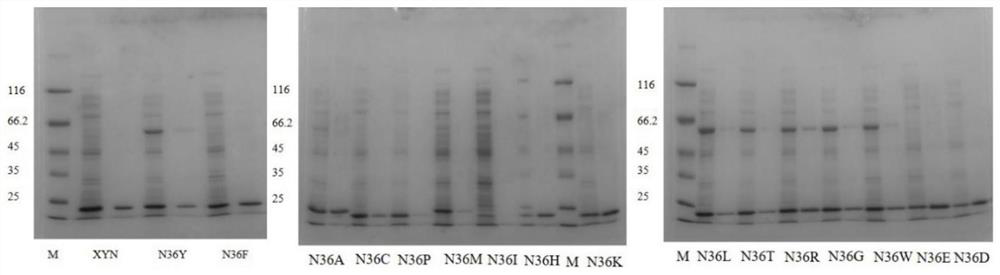
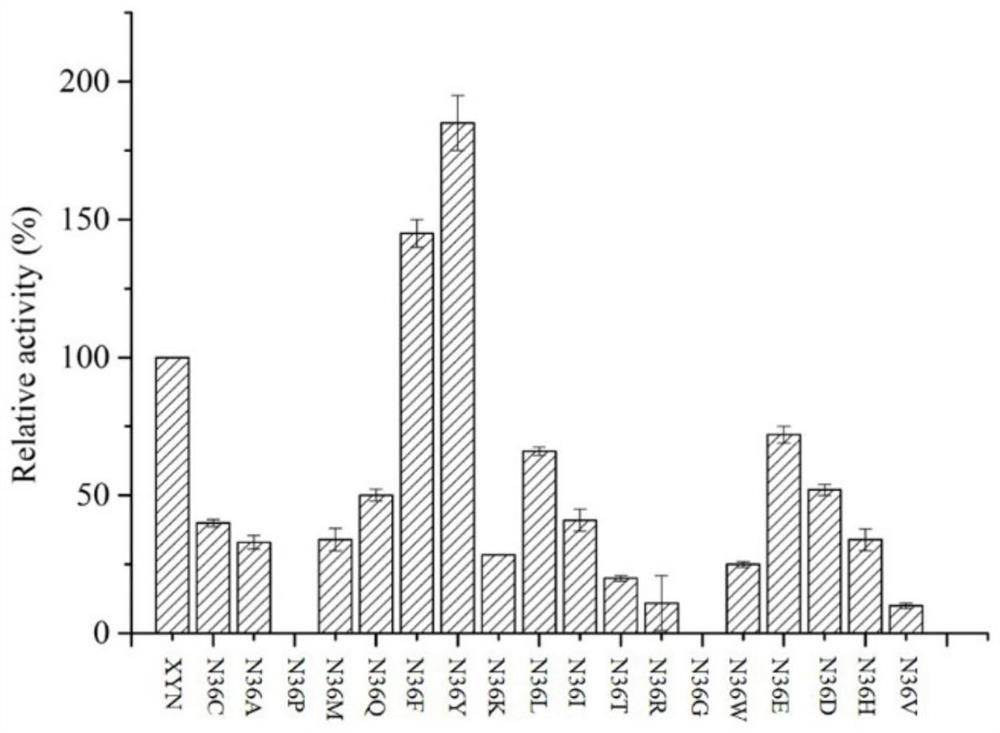
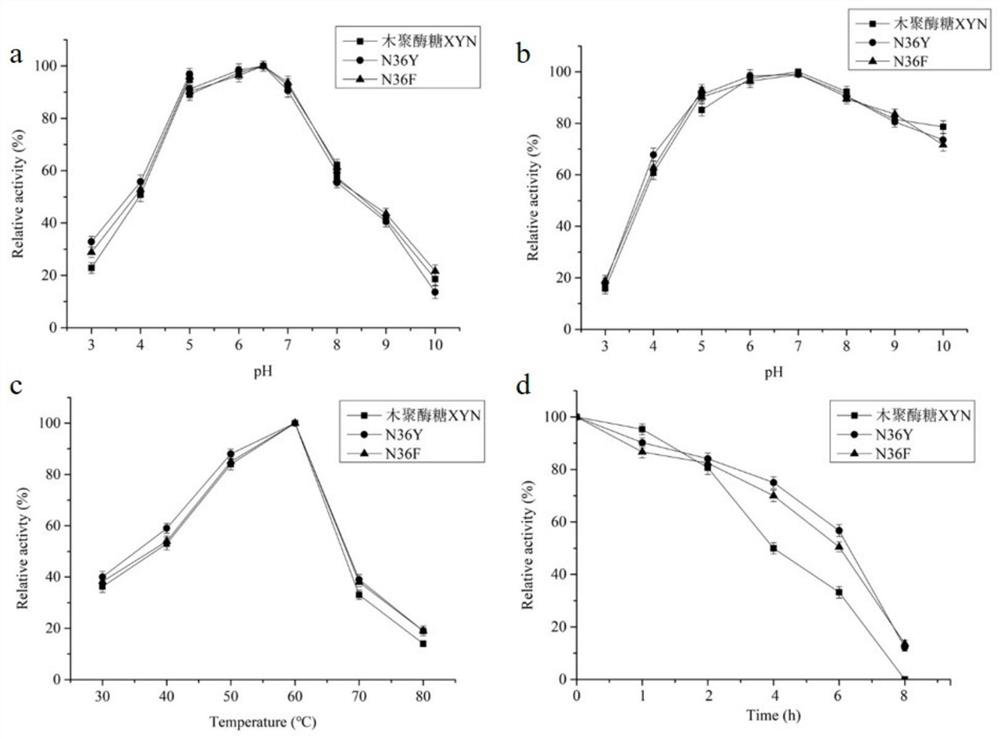
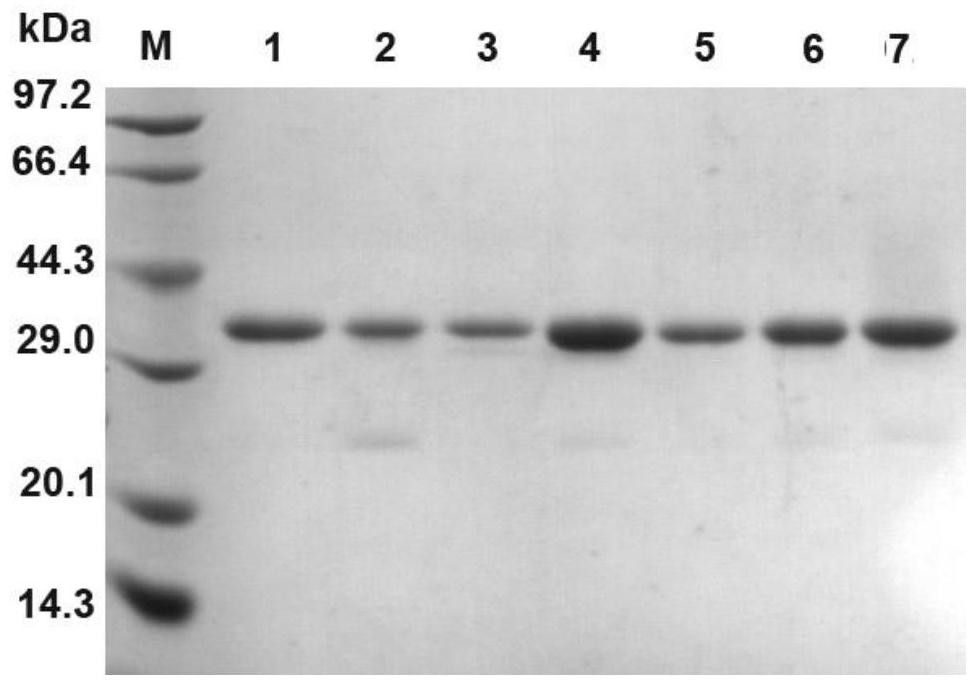
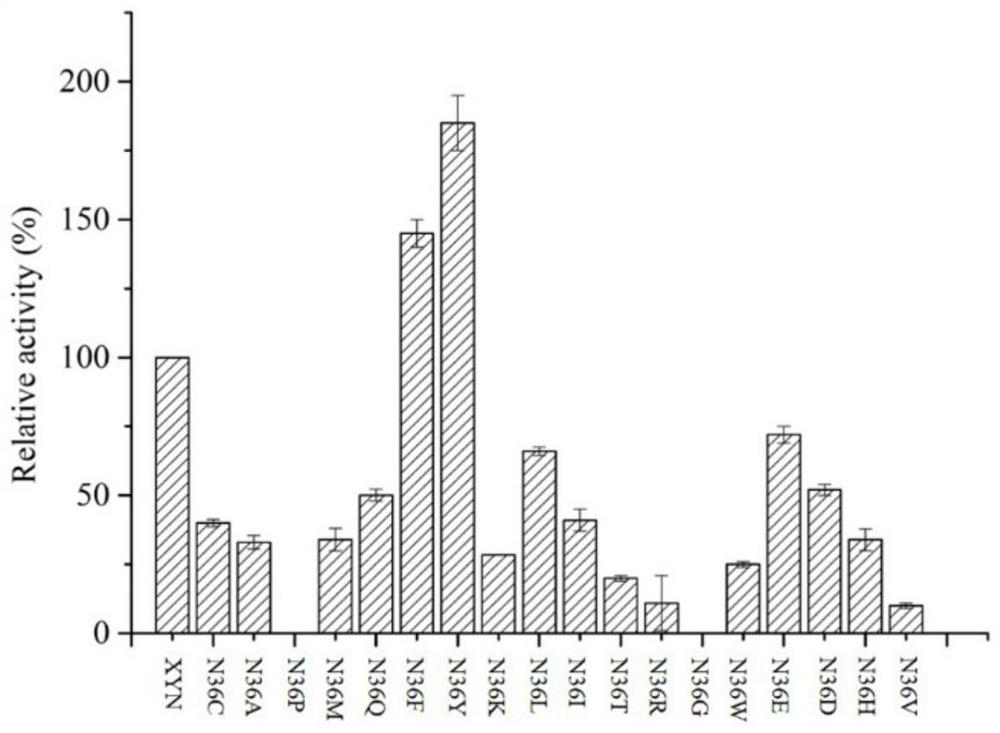
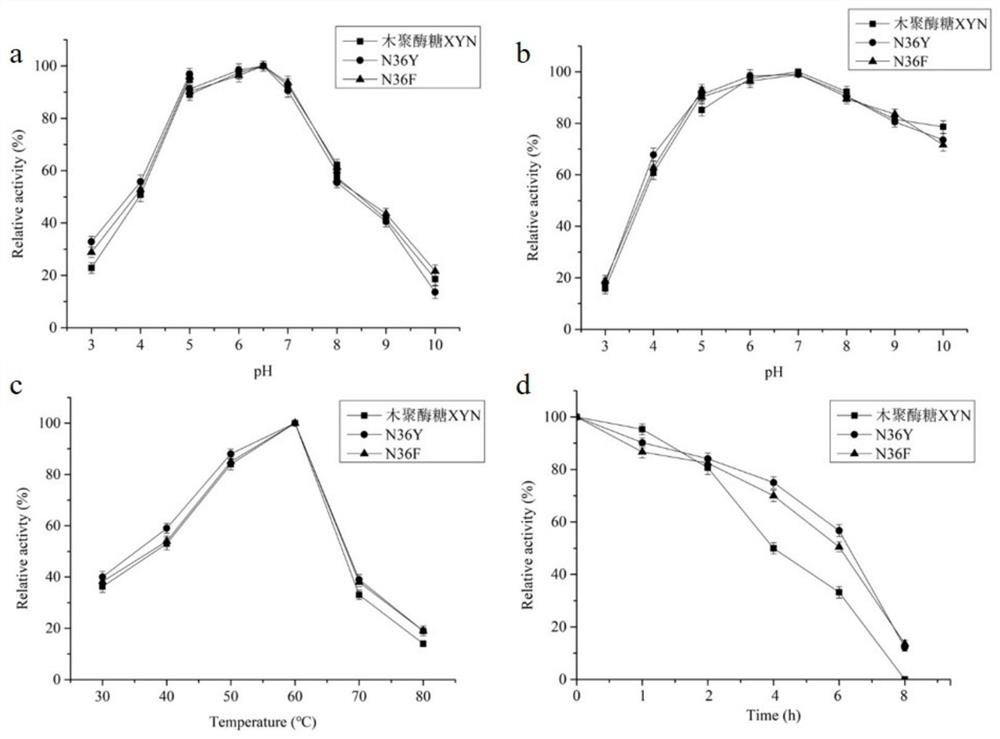
The invention relates to the field of gene engineering, and discloses a high-specific-enzyme-activity xylanase mutant and application thereof. According to the high-specific-enzyme-activity xylanase mutant, xylanase XYN from the GH11 family of Bacillus subtilis Lucky9 is used as a female parent and is expressed after being subjected to amino acid mutation by adopting a molecular biotechnology. Compared with original xylanase XYN,the mutant N36Y has the advantages that the specific enzyme activity is increased by 180% and reaches 150 U / mg, and an unexpected technical effect is achieved. The increase of the specific activity of the xylanase mutant is beneficial to reducing the production cost of xylanase and further expanding broad application of the enzyme in the field of functional sugar,so that the xylanase mutant has a wide market prospect.
Owner:NANJING UNIV OF TECH
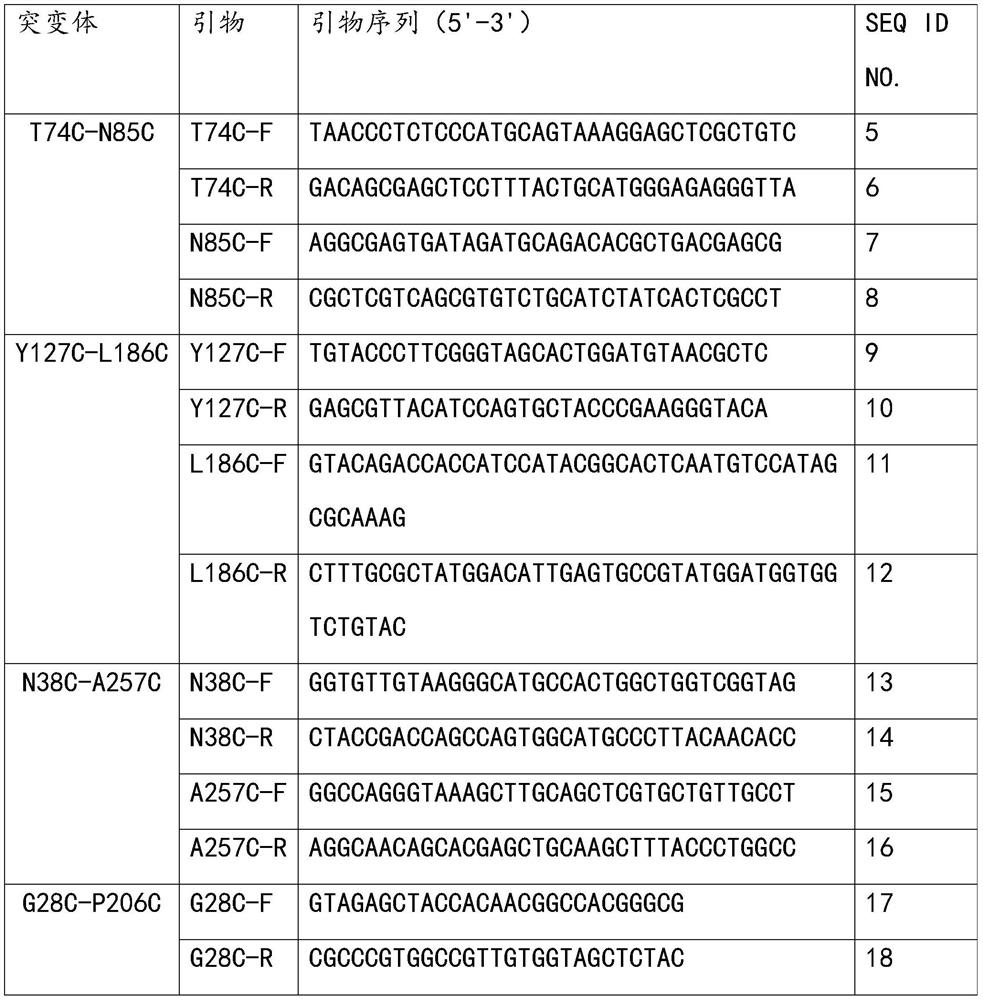
Glyceride lipase mutant G28C-P206C as well as coding gene and application thereof
ActiveCN112574975AImprove thermal stabilityHigher than vitalityFungiHydrolasesDisulfide bondingNucleotide
The invention discloses a glyceride lipase mutant G28C-P206C as well as a coding gene and application thereof. According to the glyceride lipase mutant G28C-P206C, a disulfide bond is introduced intoglyceride lipase SMG1, the amino acid sequence of the mutant is shown as SEQ ID NO.3, and the nucleotide sequence of the mutant is shown as SEQ ID NO.4. Compared with a wild type, the Tm of the glyceride lipase mutant G28C-P206C obtained by the invention is increased by 9.0 DEG C, the half-life period at 50 DEG C is increased by 64.8 times, the thermal stability is remarkably improved, and compared with the wild type, the glyceride lipase mutant G28C-P206C has better enzymatic activity and is more suitable for application in the industrial field.
Owner:SOUTH CHINA UNIV OF TECH +1
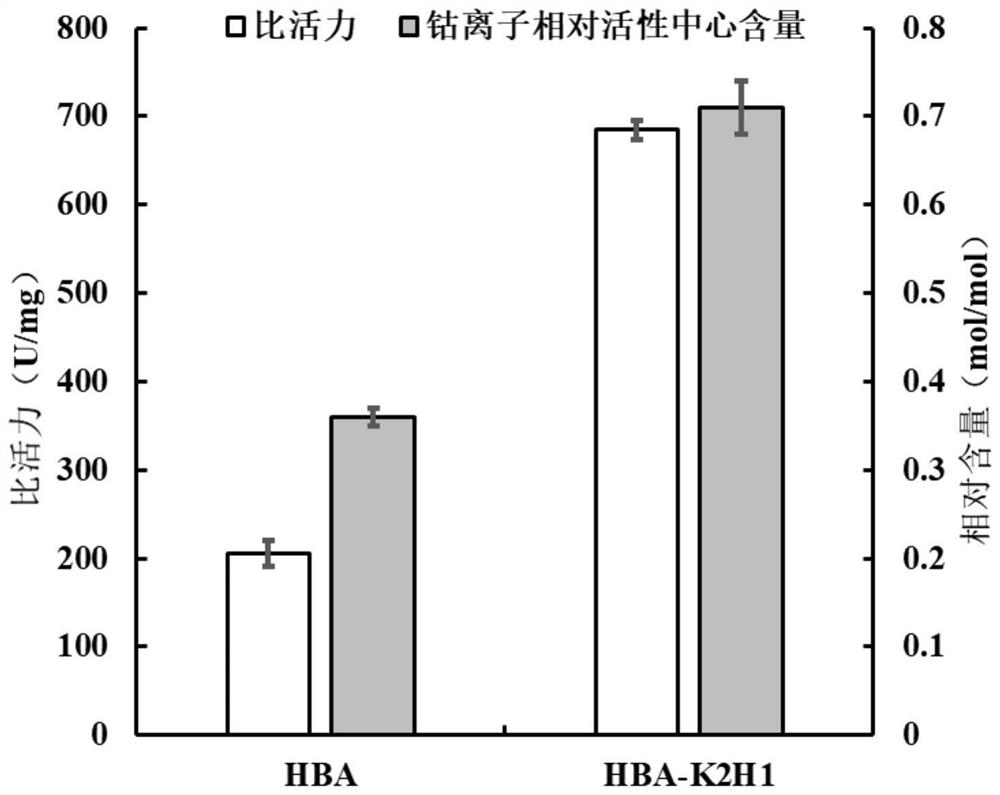
Nitrile hydratase lysine mutant HBA-K2H1, coding gene and application
ActiveCN112941062AImproved efficiency of self-modificationHigher than vitalityBacteriaMicroorganism based processesChemical compoundMutant
The invention discloses a nitrile hydratase mutant, and in particular relates to a nitrile hydratase lysine mutant HBA-K2H1 and a coding gene thereof, a plasmid and a recombinant bacterium containing the coding gene of the mutant, and application of the nitrile hydratase lysine mutant HBA-K2H1 in catalyzing an organic nitrile compound to prepare an amide compound. The amino acid sequence of the mutant HBA-K2H1 is as shown in SEQ ID NO.1, the optimum pH value of the mutant HBA-K2H1 is 8.5, and the optimum temperature of the mutant HBA-K2H1 is 35 DEG C. Compared with a wild enzyme HBA, the alkaline activity of the mutant nitrile hydratase HBA-K2H1 is improved, and the mutant nitrile hydratase HBA-K2H1 can be applied to the biotechnical fields of production of acrylamide, nicotinamide and other amide compounds with high added values through biological catalysis, chemical fiber surface modification, alkaline sewage treatment and the like.
Owner:ZHEJIANG UNIV OF TECH
Extraction and purification method of glutary-7-aminocefaphytanic acid acyltransferase
InactiveCN1428423AHigher than vitalityHydrolasesPeptide preparation methodsPurification methodsChloride
The method for extracting and purifying glutaryl-7-aminocephaphytanic acid acylase includes the following steps: firstly, using dilute hydrochloric acid to regulate pH of fermented liquor produced by fermentation, then adding calcium chloride solution, and adding chitosan, and using dilute alkali to regulate pH to 7.6-7.8, centrifuging to obtain centrifuged bacterial liquor, controlling activity of the bacterial liquor to 5-8 a / ml, adding surfactant, naturally stirring and soaking for 3-5 hr., and its purification process includes the following steps: using calcium chloride solution to downward regulate pH of soaked bacterial liquor, plate-frame filtering and collecting enzyme clear liquor, using sodium hydroxide solution to upward regulate pH of enzyme clear liquor to 7.6-80, and plate-frame filtering and collecting clear liquor.
Owner:HUNAN FLAG BIOTECHNOLOGY CO LTD
A 2,3-butanediol dehydrogenase mutant with improved enzyme activity and its construction method
ActiveCN108060145BHigher than vitalityHigh catalytic efficiencyBacteriaMicroorganism based processesBiotechnologyButanediol
The invention discloses an enzyme activity improved 2,3-butanediol dehydrogenase mutant and an establishment method thereof and belongs to the technical field of genetic engineering. According to themutant disclosed by the invention, the 52nd proline is mutated into lysine on the basis of amino acid as shown in SEQ ID No.2. The enzyme activity of the mutant disclosed by the invention is improvedby 68% when being compared with that before mutation, and the industrial application potential of the enzyme is improved.
Owner:JIANGNAN UNIV
Mutant of endoglucanase, coding gene and application thereof
ActiveCN102443576BHigher than vitalityWide operating temperature rangeFungiBacteriaAgricultural scienceReaction temperature
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
A kind of high specific enzyme activity xylanase mutant and its application
The invention relates to the field of genetic engineering, and discloses a xylanase mutant with high specific enzyme activity and application thereof. The invention uses the xylanase XYN of the GH11 family derived from Bacillus subtilis Lucky9 as the mother parent, carries out amino acid mutation and expression by molecular biological technology. Compared with the original xylanase XYN, the specific enzyme activity of the mutant N36Y is increased by 180%, reaching 150U / mg, which has achieved unexpected technical effects. The improvement of the specific activity of the xylanase mutant in the invention is beneficial to reduce the production cost of the xylanase, further expands the wide application of the enzyme in the field of functional sugars, and has broad market prospects.
Owner:NANJING TECH UNIV
Preparation method of lactococcus lactis product expressing phenylalanine ammonia lyase
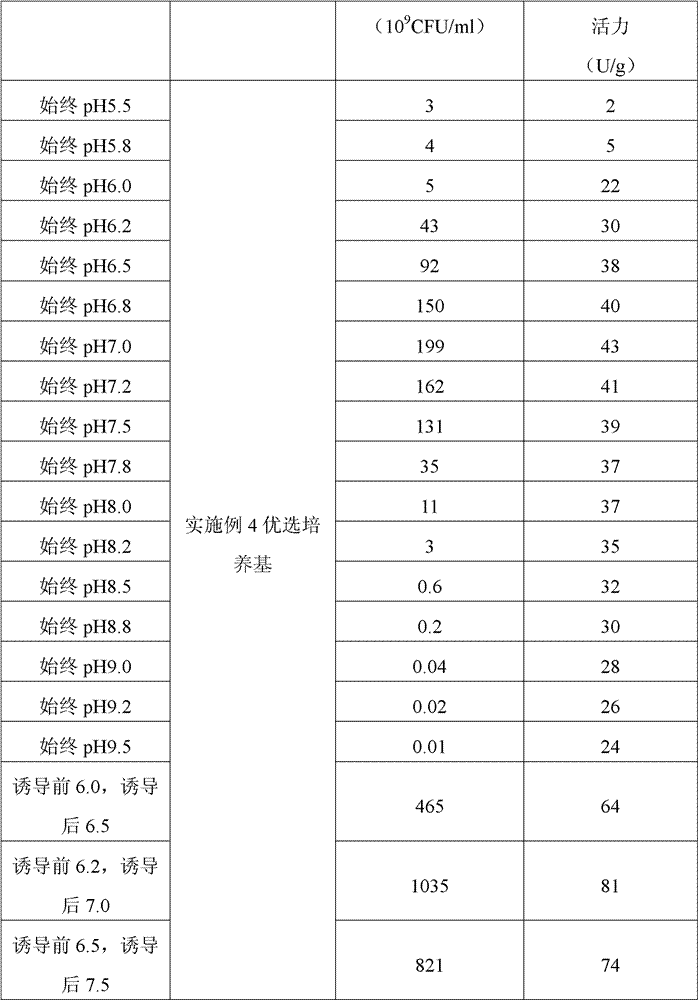
ActiveCN102358889BHigher than vitalityBacteriaMicroorganism based processesStaphylococcus lactisBiochemistry
The invention belongs to the field of biological medicine, and relates to a preparation method of a lactococcus lactis product expressing phenylalanine ammonia lyase, and the method orderly comprises the following steps: 1) obtaining a gene which expresses phenylalanine ammonia lyase; 2) connecting the gene which expresses phenylalanine ammonia lyase with a expression vector which is suitable forlactococcus lactis transformation so as to form a recombinant expression vector; 3) transforming competent lactococcus lactis by the recombinant expression vector; 4) screening to obtain a lactococcus lactis strain which highly expresses phenylalanine ammonia lyase; 5) fermenting the lactococcus lactis and inducing the expression of phenylalanine ammonia lyase; 6) drying the obtained fermented strain; wherein the pH of the fermentation liquid is adjusted to be 6.0-6.5 before the induction of fermentation in step 5); the pH is adjusted to be 6.5-7.5 after the induction; the strain is continued to be cultured till the fermentation is finished. With the technical scheme of the invention, the living bacteria number of the obtained lactococcus lactis and the specific activity of phenylalanine ammonia lyase are greatly increased, wherein the former reaches up to above 1012 CFU / ml, and the later reaches up to above 80 U / g.
Owner:BEIJING TRI PRIME GENE PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com