Low-temperature beta-xylosidase mutant with improved thermal stability and specific activity and encoding gene and application thereof
A technology of xylosidase and thermal stability, applied in the field of genetic engineering and enzyme engineering, can solve the problems of poor thermal stability of low-temperature xylosidase, unfavorable application of wild-type low-temperature β-xylosidase, and low specific activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] Cloning of embodiment 1 mutant enzyme and wild enzyme expression gene
[0091] The present invention uses the low-temperature xylosidase AX543 as the parent, adopts the method of Overlap-PCR, uses the primers in Table 1 to mutate and express the low-temperature xylosidase AX543, and its gene sequence is as shown in SEQ ID NO.4 / SEQ ID NO.5 / SEQ ID NO.6 shown.
[0092] Table 1. PCR-specific primers for β-xylosidase mutants G110S, Q201R and loop2
[0093]
Embodiment 2
[0094] Expression and purification of embodiment 2 mutant enzyme and wild enzyme in Pichia pastoris
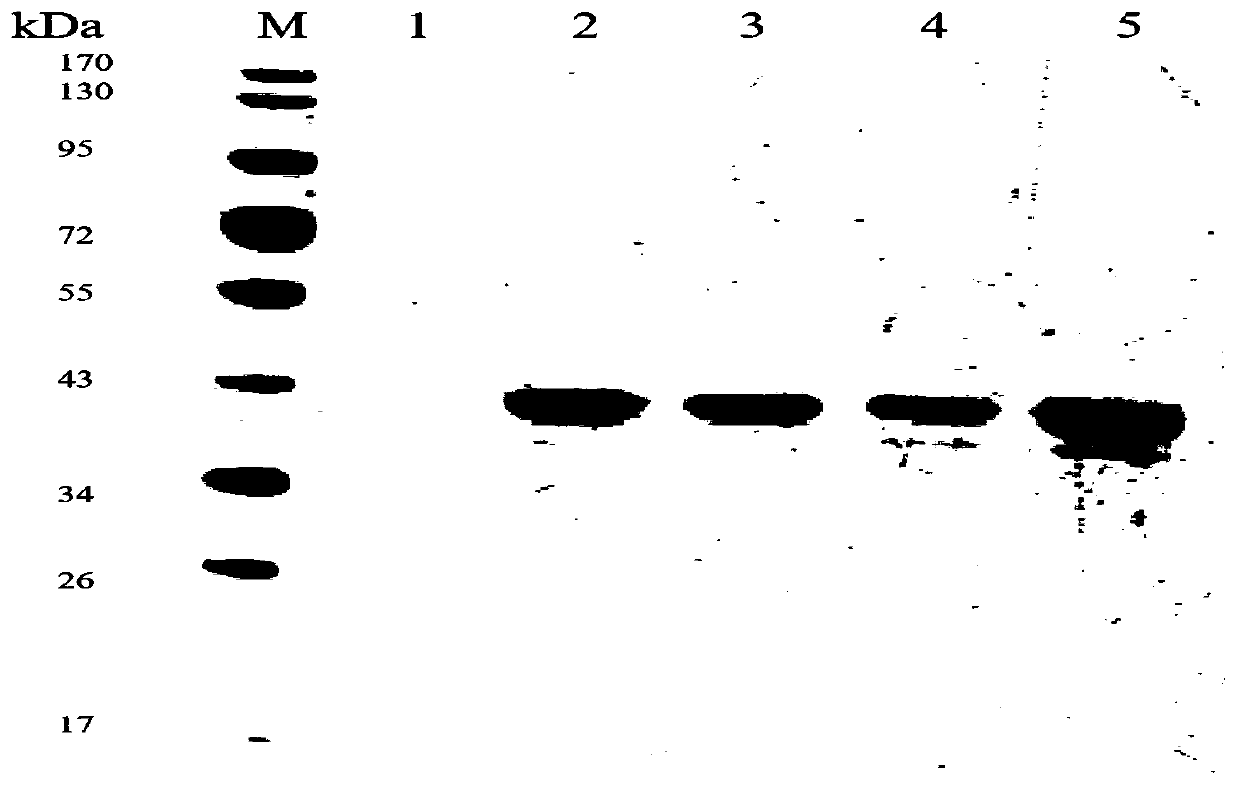
[0095] The PCR products of β-xylosidase mutants G110S, Q201R, and loop2 and the expression vector pPIC9 were subjected to double enzyme digestion (EcoRI+XholⅠ), and the cut gene fragments were connected to the vector to obtain a low-temperature protein with high thermal stability and specific activity. The recombinant plasmids of β-xylosidase mutants G110S, Q201R and loop2 were linearized and transformed into Pichia pastoris GS115 competent cells by electric shock to obtain recombinant yeast strains GS115 / G110S, GS115 / Q201R and GS115 / loop2.
[0096] Pick the monoclonal colony on the MD plate, and use a toothpick stained with bacteria to spot it on another numbered MD plate and a 96-well plate containing 1mL of BMGY medium, respectively. While culturing the MD plate at 30°C, Cultivate a 96-well plate containing 1mL of BMGY medium at 30°C and 220r / min for 48h; after 48h of cultu...
Embodiment 3
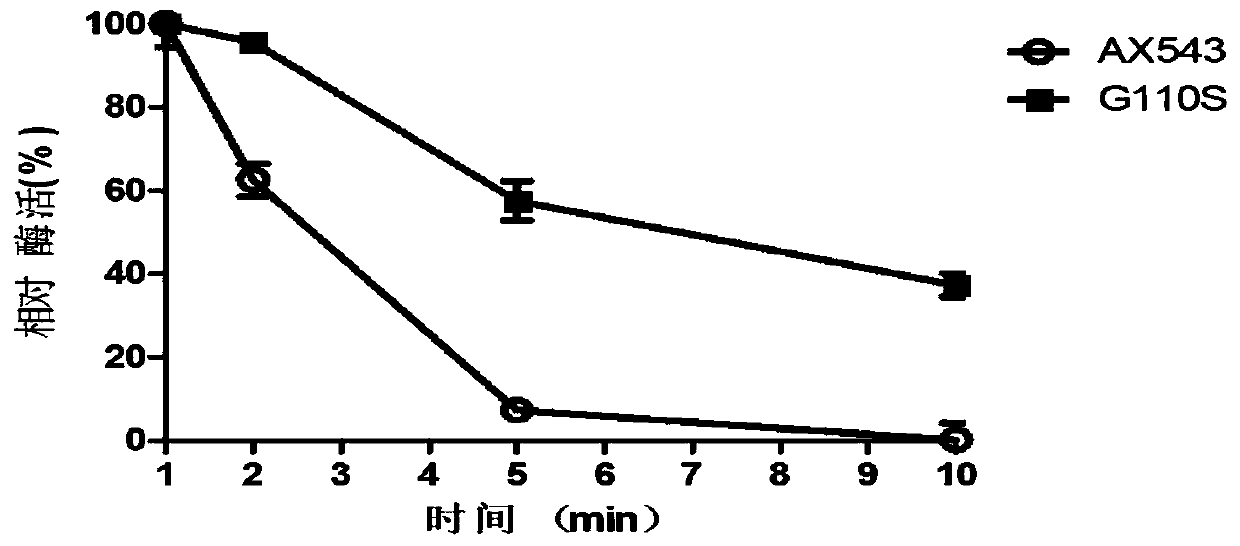
[0098] Activity Analysis of Embodiment 3 Mutant Enzyme and Wild Enzyme
[0099] One enzyme activity unit (U) is defined as the amount of enzyme required to decompose the substrate pNPX to produce 1 μmol p-nitrophenol (pNP) per minute under certain reaction conditions.
[0100] Determination of β-xylosidase activity: Add 150 μL citric acid-disodium hydrogen phosphate buffer solution to 250 μL substrate pNPX, add 100 μL enzyme solution (2U / ml) after preheating at 20°C for 2 minutes, react at 20°C for 10 minutes, add 1.5mL, 1mol / L Na 2 CO 3 Solution termination reaction, OD 405 Measure its absorbance.
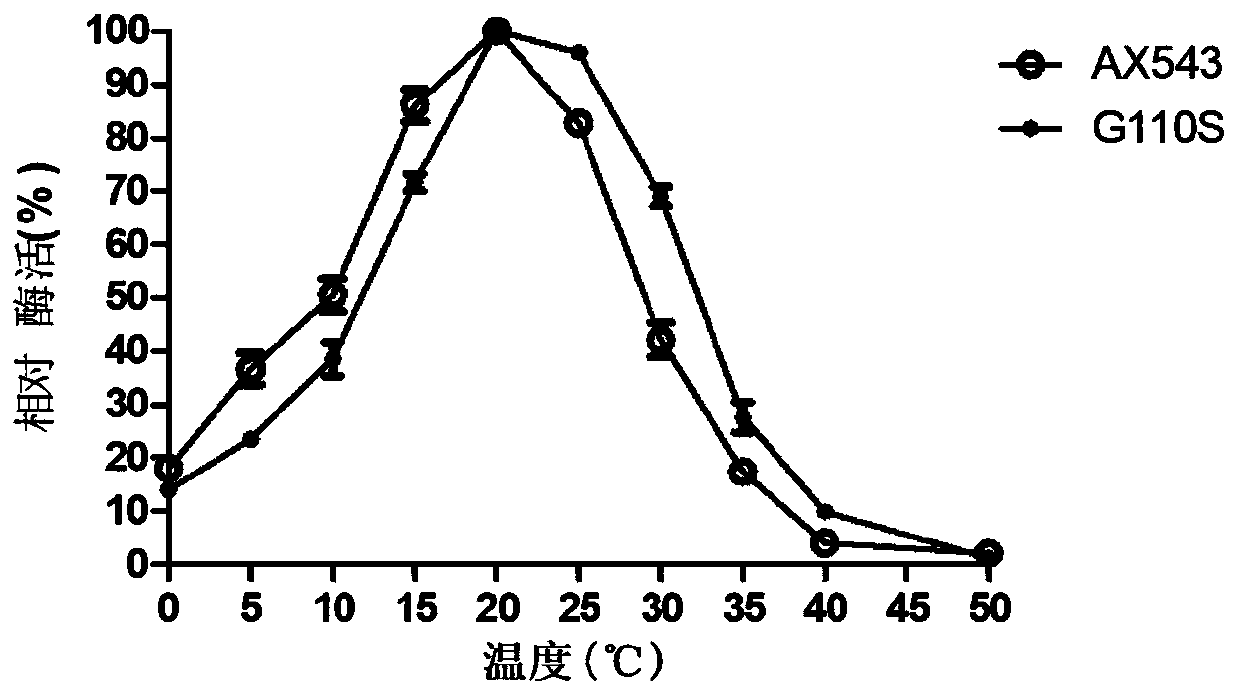
[0101] Optimum temperature for β-xylosidase: 2mM pNPX as substrate, 150μL 0.2mol / L citric acid-disodium hydrogen phosphate buffer (pH6.0) was added to 250μL substrate, and the substrate and buffer The mixture was preheated in a water bath at 0°C-50°C for 2 minutes, then 100 μL of enzyme solution (2U / ml) was added, reacted at 0°C-50°C for 10 minutes, and 1.5 mL of 1M Na 2 CO ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific vitality | aaaaa | aaaaa |
| Specific vitality | aaaaa | aaaaa |
| Specific vitality | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com