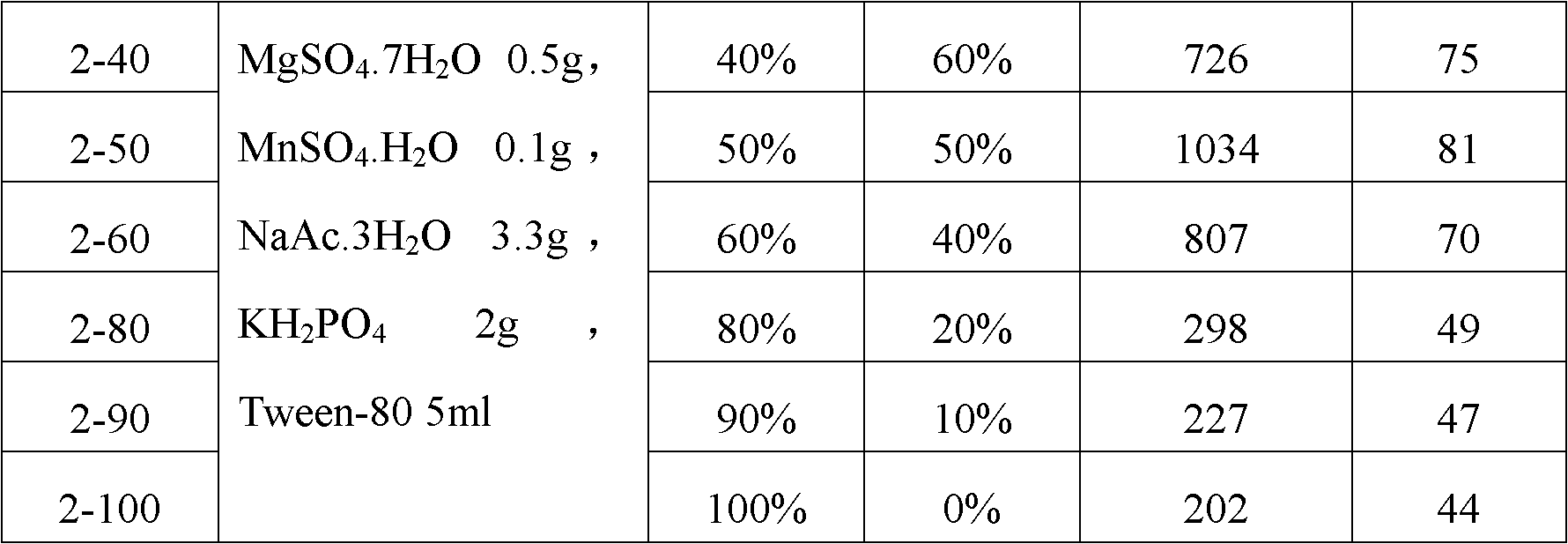Preparation method of lactococcus lactis product for expressing phenylalanine ammonialyase
A technology of phenylalanine ammonia lyase and Lactococcus lactis, which is applied in the field of preparation of Lactococcus lactis products, can solve the problems of limiting practical clinical application, high cost, and low total level of PAL
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
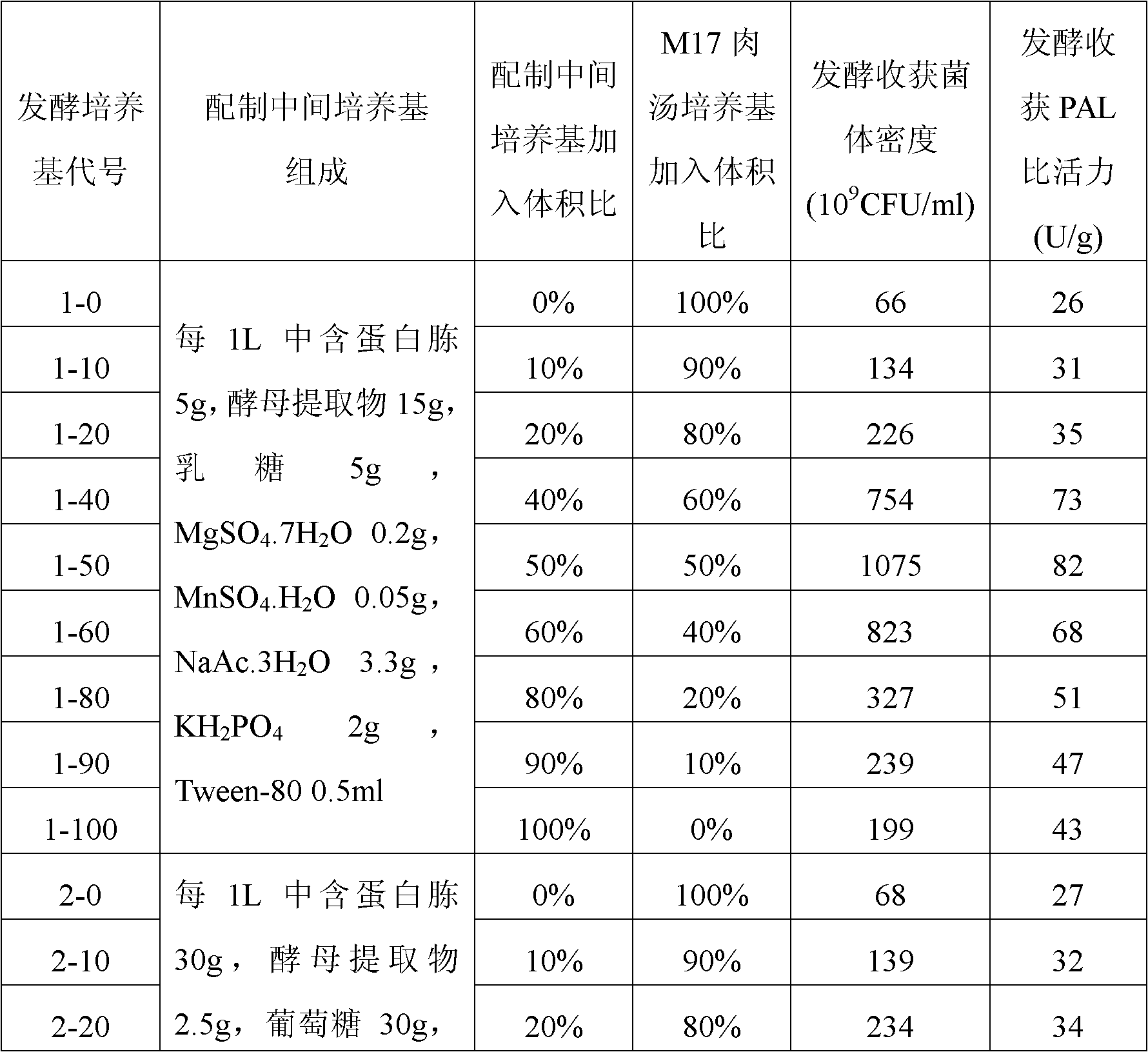
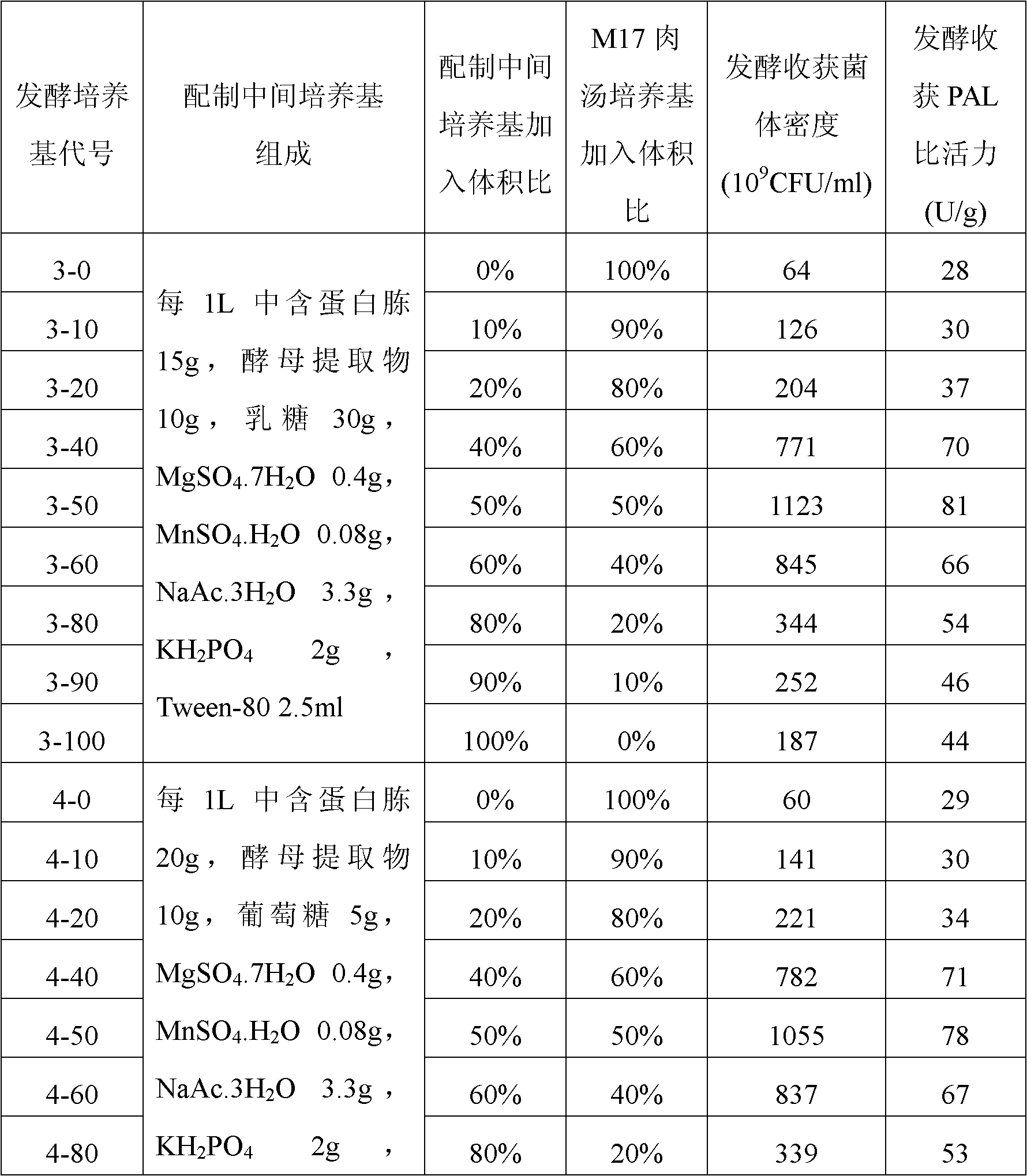
[0027] Example 1: Construction of Lactococcus lactis engineering bacteria expressing PAL by utilizing the preferred codons of Lactococcus lactis human synthetic PAL gene
[0028] Artificially synthesized literature "Food-grade expression of artificially synthesized phenylalanine deaminase gene in Lactococcus lactis" (Jia Xingyuan, Chen Xing, Su Chang, etc., Chinese Journal of Microbiology and Immunology, Volume 26, No. 1, January 2006 The sequence of the PAL gene using the preferred codons of Lactococcus lactis as shown in Figure 1 in Issue 23-26), labeled as PAL art, DNA sequencing after ligation to the T vector was verified. The PAL art There are XbaI and NcoI restriction sites at the 5' end and 3' end, respectively.
[0029] NcoI and XbaI double enzyme digestion containing PAL art Complete PAL after fragmenting the T vector art The gene fragment was then ligated with the linear plasmid obtained by double digestion of pNZ8149 with NcoI and XbaI under the action of T4DNA ...
Embodiment 2
[0030] Example 2: PAL art Screening and Identification of Recombinant Expression Plasmids
[0031] Screening and identification were carried out by restriction enzyme cleavage map method and PAL enzyme activity detection method, respectively.
[0032] The restriction map method uses NcoI and XbaI double enzyme digestion, and the electrophoresis results show that the recombinant plasmid is cut into two bands of 2.8kb and 1.9kb, while the plasmid pNZ8149 is cut into two bands of about 1.9kb and 650bp, which are in line with the expected The number of bands is consistent with the size and position, and it can be confirmed that the recombinant plasmid has a 2.2kb PAL art Fragment insertion.
[0033] PAL enzyme activity detection method Take 400μl of bacterial solution, add an equal amount of 5% perchloric acid and 10mmol / L phenylalanine, adjust the pH to 8.8 with 0.1mol / L borate buffer, react at 30°C for 1 hour, and take after centrifugation at 12000rpm The supernatant was filt...
Embodiment 3
[0034] Example 3: Using the parsley PAL gene to construct Lactococcus lactis engineering bacteria expressing PAL
[0035] Extract total RNA from mechanically damaged parsley stems and leaves by conventional methods, and prepare the cDNA of PAL by RT-PCR technology, wherein the specific primers used are:
[0036] Upstream primer T1: 5′-TACGATATCAGGAACTTGGTAACAAT-3′,
[0037] Downstream primer T2: 5'-CTAAGATCTCATGCATFTCAGTTAACA-3'.
[0038] The resulting cDNA fragment was digested with EcoRV and BglII to obtain a PAL cDNA fragment with cohesive ends. Then, the expression vector pNZ8149 was digested with EcoRV and BglII, and the digested fragment was ligated with the PAL cDNA fragment under the action of T4DNA ligase to obtain the recombinant plasmid pNZ8149-PAL.
[0039] Referring to the electroporation conditions of Example 1, the recombinant plasmid pNZ8149-PAL was transformed into competent Lactococcus lactis NZ3900. The screening and identification methods of the pNZ8149-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com