High-specific-enzyme-activity xylanase mutant and application thereof
A xylanase mutation and xylanase technology, which is applied in the field of high specific enzyme activity xylanase mutants and can solve the problems of poor stability and inability to meet industrial applications and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1 Mutation of xylanase mutant gene
[0023] In order to improve the activity of xylanase XYN (amino acid sequence as shown in SEQ ID NO.1, nucleic acid sequence: SEQ ID NO.2) derived from the GH11 family of Bacillus subtilis Lucky9, 4 xylanases with xylanase were selected by substrate docking. Glycan chain binding related sites, it was found that 36N had the most significant effect on enzyme activity. Therefore, a large number of mutations were screened at this site by directed evolution technology, and the following mutation primers were designed:
[0024]
[0025]
[0026] Using the xylanase XYN gene as a template, referring to Vazyme biological products and operating manuals, the site-directed mutation sequence was amplified from the whole plasmid. After digestion with Dpn I, the PCR product was transformed into Escherichia coli competent cells E.coli BL21(DE3) by heat shock method, and spread on LB agar containing 100 μg / ml kanamycin sulfate On th...
Embodiment 2
[0027] Embodiment 2 The acquisition of xylanase and mutant enzyme
[0028] Inoculate the mutants constructed in Example 1 and the original enzyme XYN into 50 mL of LB liquid medium, add kanamycin sulfate to make the final concentration 100 μg / mL, culture at 180 rpm, 37°C overnight; inoculate with 2% Inoculate the overnight cultivated seed liquid into fresh 50mL LB liquid medium, 180rpm, 37 ℃ constant temperature culture to OD 600 When it is 0.6-1.0, add inducer IPTG (isopropyl-β-D thiogalactopyranoside) (final concentration 0.1mmol / L), and induce expression at 25°C for 24h.
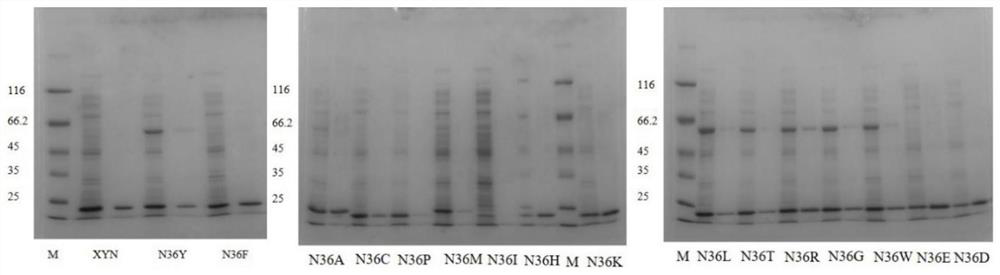
[0029] Get the fermented liquid of induced expression, centrifuge at 12000rpm for 20min, discard the supernatant, then wash with 50mM Na 2 HPO 4 -KH 2 PO 4 (pH 7.0) to buffer and resuspend the bacteria, then ultrasonically break, and perform SDS-PAGE electrophoresis detection. The concentration of the stacking gel is 4%, and the concentration of the separating gel is 12.5%. React for 5 min for loadin...
Embodiment 3
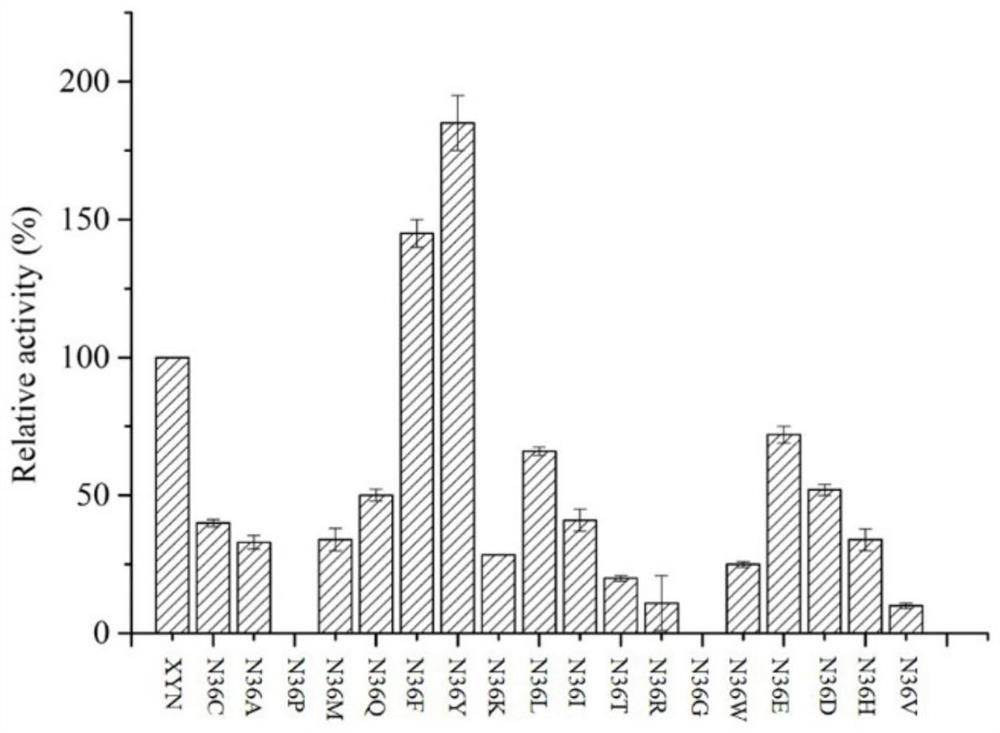
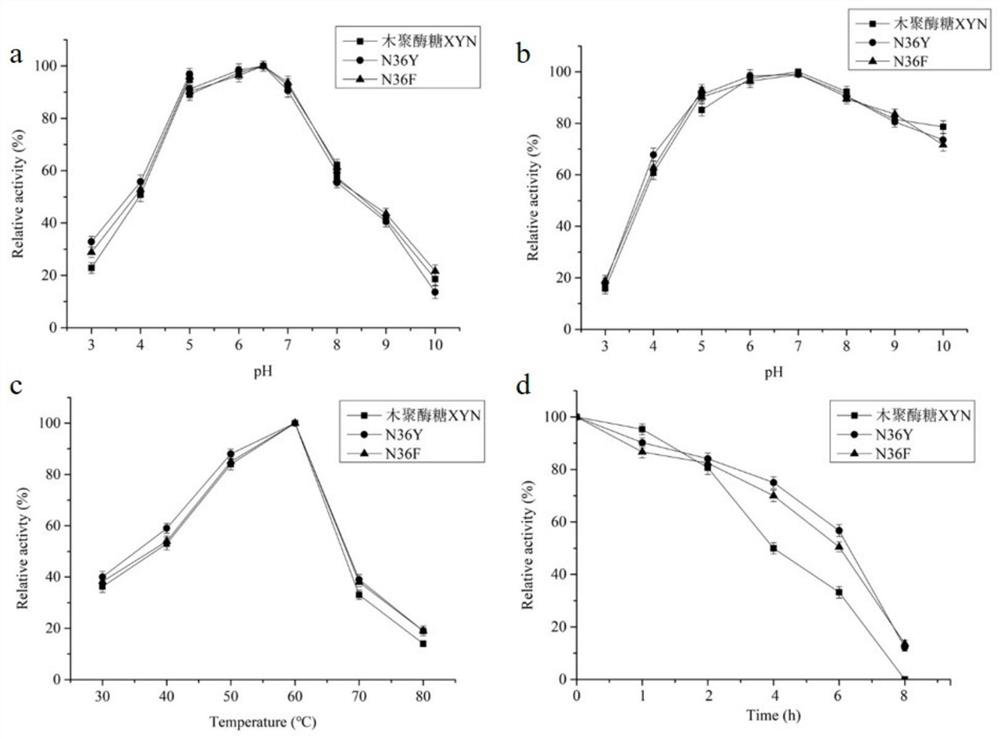
[0041] Example 3 Screening procedure for the optimal mutant of xylanase XYN
[0042] Method for Determination of Hydrolytic Activity of Xylanase XYN Mutant
[0043] Definition of enzyme activity unit: One enzyme activity unit is defined as the amount of enzyme required to produce 1 mmol of reducing sugar from the substrate per minute under the conditions of 60°C and pH 7.0.
[0044] Xylanase (Endo-glucanase): Accurately weigh 1g of beech wood xylan dissolved in 100mL of Na 2 HPO 4 -KH 2 PO 4 Buffer (50mM, pH 7.0), stir and mix, accurately pipette 1.0mL into the test tube as the enzyme reaction substrate, preheat at 60°C for 5 minutes, add 0.5mL of protease solution that has been properly diluted, and put it in a water bath shaker at 60°C for reaction After 10 minutes, 3 mL of DNS (3,5-dinitrosalicylic acid) reagent was added, reacted in a boiling water bath for 5 minutes, then rapidly cooled to room temperature, and the absorbance value at a wavelength of 540 nm was measur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com