Nitrile hydratase lysine mutant HBA-K2H2R, coding gene and application
A nitrile hydratase lysine, encoding gene technology, applied in the application, genetic engineering, plant gene improvement and other directions, can solve the problem of artificially regulating the self-modification efficiency of nitrile hydratase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Site-directed mutagenesis of nitrile hydratase HBA
[0039] 1) Extract the recombinant plasmid pET-28-HBA containing the nitrile hydratase HBA gene from the recombinant Escherichia coli HBA according to the instructions of the plasmid mini-extraction kit from Axygen Company. The amino acid sequence of wild-type nitrile hydratase HBA is shown in SEQ ID NO.3.
[0040] 2) According to the nucleotide sequence of wild-type nitrile hydratase HBA (SEQ ID NO.4), design multi-site site-directed mutagenesis primers 5'-CAAACATGGTTTCGGCAAGATCATTCGTGAGAAACATGAGCCGCTGTTCC-3'(SEQ ID NO.5), 5'-GATCTTGCCGAAACCATGTTTGCCACCCAGGTCGTGG-3' (SEQ ID NO. 6), 5'-CAGCAGACCAAACGCGCGACGTTCCC AGTC-3' (SEQ ID NO. 7) and 5'-CGCGCGTTTGGTCTGCTGATTGGCACCGCGGGTC-3' (SEQ ID NO. 8).
[0041] 3) According to the instructions of the Fast MultiSite Mutagenesis System Kit of Beijing Quanshijin Biotechnology Co., Ltd., the recombinant plasmid pET-28-HBA obtained in step 1 was used as a template, and ...
Embodiment 2
[0043] Embodiment 2: Enzyme preparation of nitrile hydratase mutant HBA-K2H2R and wild-type HBA
[0044] 1) Recombinant strains containing mutant HBA-K2H2R and wild-type HBA were inoculated in LB (50 μg / mL Kan) medium at an inoculum size of 0.1%, respectively, and cultured at 37° C. and 180 rpm for 16 hours with shaking.
[0045] 2) Inoculate the 1% inoculum of the activated bacterial liquid overnight into fresh LB (containing 50 μg / mL Kan) culture liquid, culture at 37°C with shaking at 180 rpm for 3 h (OD 600nm reach 0.6-1.0).
[0046] 3) Add IPTG (isopropylthiogalactopyranoside) at a final concentration of 0.5 mM for induction, and culture at 20° C. with shaking at 150 rpm for about 20 h.
[0047] 4) Centrifuge at 8000rpm for 5min, collect the cells and suspend the cells with PBS buffer.
[0048] 5) Ultrasonic disrupt the bacteria in a low-temperature ice-water bath. Ultrasonic crushing conditions are: power 300W, 5s on, 10s off, temperature 4°C, effective crushing time ...
Embodiment 3
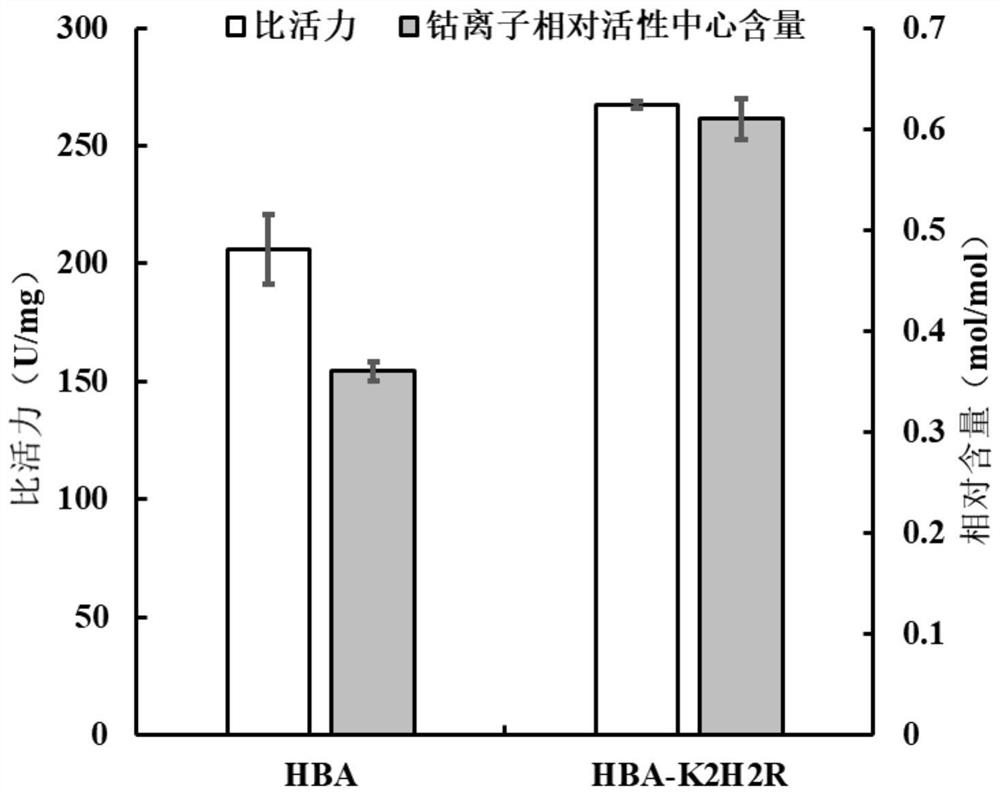
[0051] Embodiment 3: The property determination of the purified enzyme of nitrile hydratase mutant HBA-K2H2R and wild-type HBA
[0052] 1) Activity analysis of mutant HBA-K2H2R and wild-type HBA purified enzymes
[0053] The substrate 3-cyanopyridine was dissolved in 50mM boric acid-borax buffer solution (pH 8.0) to make the final concentration 10mM. After the reaction solution was preheated at 40°C for 5min, the final concentration of pure enzyme solution was added to make it final. The concentration was 7.2 μg / mL, and reacted at 40° C. for 10 min, and then 20 μL of 5M concentrated HCl was added to terminate the reaction. After removing impurities by filtration with a 0.22 μm filter membrane, the amount of the product 3-pyridinecarboxamide formed was detected by HPLC.
[0054] The detection conditions of HPLC are as follows: Agilent InfinityLab Poroshell 120 EC-C18 column (4.6×150mm, 4μm) chromatographic column is used, the column temperature is 36°C, the mobile phase is 10%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com