Pullulanase mutant and preparation method thereof
A pullulanase and mutant technology, applied in the fields of botanical equipment and methods, biochemical equipment and methods, DNA preparation, etc., can solve the problems that thermal stability cannot fully meet the saccharification process, low catalytic efficiency, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: Recombinant bacteria construction
[0021] According to the pulA gene sequence registered on NCBI (NCBI accession number: AX203845), the starch debranching enzyme gene sequence pulA was synthesized by chemical total synthesis. The plasmid used to construct the expression vector in E. coli is pET20b(+) with T7 promoter. The pET20b(+) plasmid and the plasmid containing the pulA gene were subjected to NcoⅠ and HindⅢ double enzyme digestion respectively. After the digestion products were recovered by tapping the rubber, they were ligated with T4 ligase, and the ligated products were transformed into E.coli JM109 competent cells and cultured at 37°C At 8 hours, the transformants were picked and cultured with shaking in LB containing 100 mg / L ampicillin liquid, the plasmid was extracted, and the expression plasmid pulA / pET20b(+) was obtained for enzyme digestion verification.
[0022] The plasmid pulA / pET20b(+) was transformed into E.coli BL21(DE3) host bacteri...
Embodiment 2
[0023] Example 2: Preparation of mutants.
[0024] (1) Single mutation
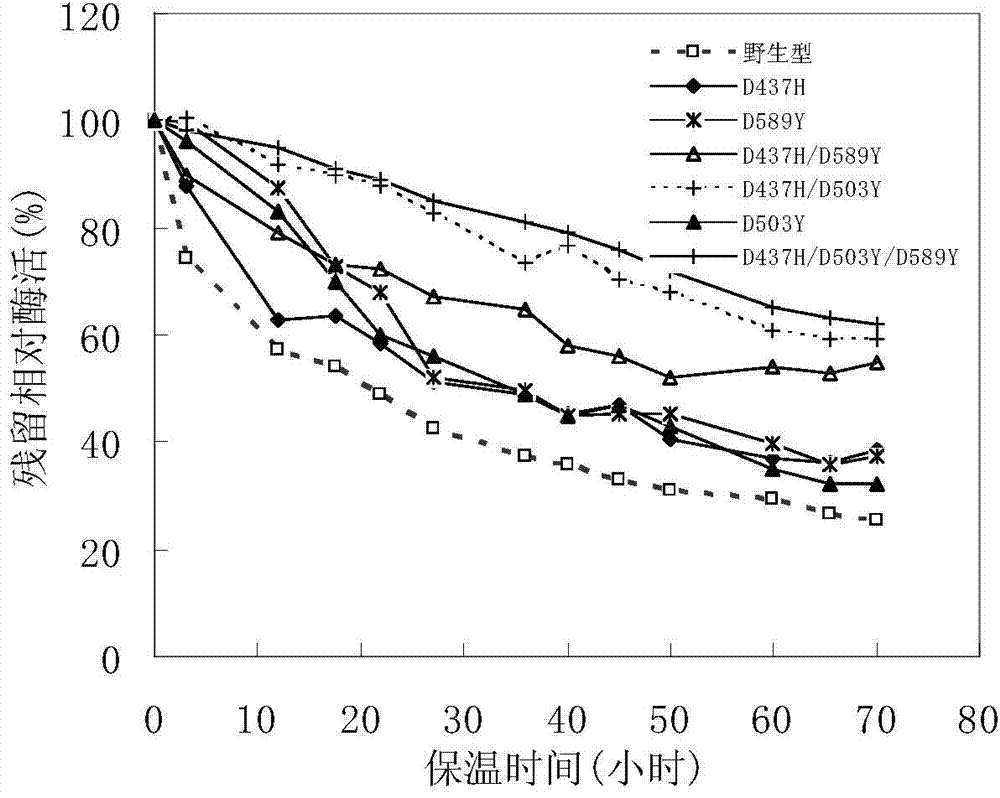
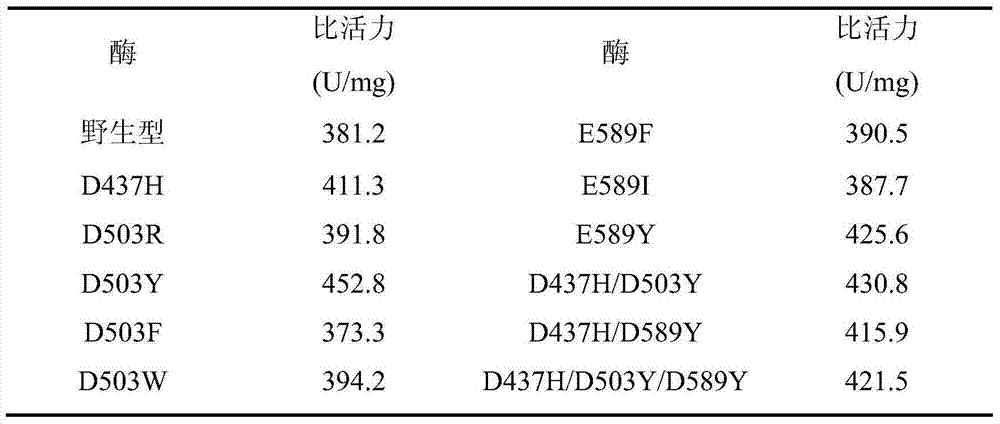
[0025] Six single mutant enzymes D437H, D503R, D503F, D503W, D503Y and E589Y of pullulanase derived from B.deramificans:
[0026] Based on the comparison and analysis of pullulanase sequences from different sources, the structure of the pullulanase protein from Bacillus demycotina was simulated and rationally analyzed, combined with the results of the thermodynamic analysis of pullulanase, the debranching Bacillus spp. Three amino acid sites (Asp437, Asp503 and Glu589) in the Bacillus pullulanase molecule that have potential effects on the thermostability of the enzyme.
[0027] The aspartic acid (Asp) at position 437 in the pullulanase gene was mutated into histidine (His), named D437H; the aspartic acid (Asp) at position 503 in the pullulanase gene was ) were mutated into arginine (Arg), phenylalanine (Phe), tryptophan (Trp) or tyrosine (Tyr) respectively named D503R, D503F, D503W and D503Y; The glut...
Embodiment 3
[0078] Example 3: This example illustrates an enzyme activity assay.
[0079] 1) Enzyme activity assay method
[0080] The enzyme activity of pullulanase was determined by 3,5-dinitrosalicylic acid (DNS method). Under certain conditions, pullulanase catalyzes the hydrolysis of pullulan sugar to generate reducing sugar, and 3,5-dinitrosalicylic acid is reduced to a brownish-red amino complex after being heated together with the reducing sugar solution. The depth of its color within the range is proportional to the amount of reducing sugar, so the colorimetry can be performed at a wavelength of 540nm to calculate the enzyme activity. Definition of enzyme activity unit: Under the above conditions, the amount of enzyme that catalyzes the production of 1 μmol of glucose per minute is regarded as an activity unit.
[0081] Enzyme activity assay steps:
[0082] A. Preheating: Take 2ml of 0.5% pullulan solution (50mM pH4.5 acetic acid buffer) in a test tube and place it in a 60°C w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com