Alkaline protease mutant PROK-M with increased specific activity and thermal stability, and coding gene and applications thereof
A technology of thermostability and protease, which is applied in the field of agricultural biology, can solve the problems of enzyme activity and thermostability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1 Preparation of alkaline protease mutant recombinant vector pPIC9r-PROK-M
[0051] The biological source of the wild-type alkaline protease of the present invention is a fungus derived from Rosa phyllosa (Parengyodontium album).
[0052] The sequence fragment of the alkaline protease wild type without the signal peptide was cloned into the expression vector pPIC-9r, and the recombinant vector was named pPIC9r-PROK; using the recombinant vector pPIC9r-PROK as a template, it was amplified by primers carrying mutation sites, A recombinant vector carrying the mutant sequence was obtained and named pPIC9r-PROK-M.
[0053] The same method was used to construct the recombinant expression vector of the gene encoding the signal peptide sequence.
[0054] Table 1 alkaline protease mutant PROK-M specific primers
[0055]
Embodiment 2
[0056] Embodiment 2 prepares alkaline protease mutant
[0057] The obtained recombinant plasmid pPIC9r-PROK-M was transformed into Pichia pastoris GS115 to obtain recombinant yeast strain GS115 / PROK-M. Take the GS115 strain containing the recombinant plasmid, inoculate it in a 1L Erlenmeyer flask with 300mL of BMGY medium, place it at 30°C, and culture it on a shaker at 220rpm for 48h; then centrifuge the culture solution at 3000g for 5min, discard the supernatant, and use 100mL of 0.5% methanol for precipitation. The BMMY medium was resuspended, and placed again at 30°C, 220rpm to induce culture. Add 0.5 mL of methanol every 12 hours to keep the concentration of methanol in the bacterial solution at 0.5%, and take the supernatant for enzyme activity detection.
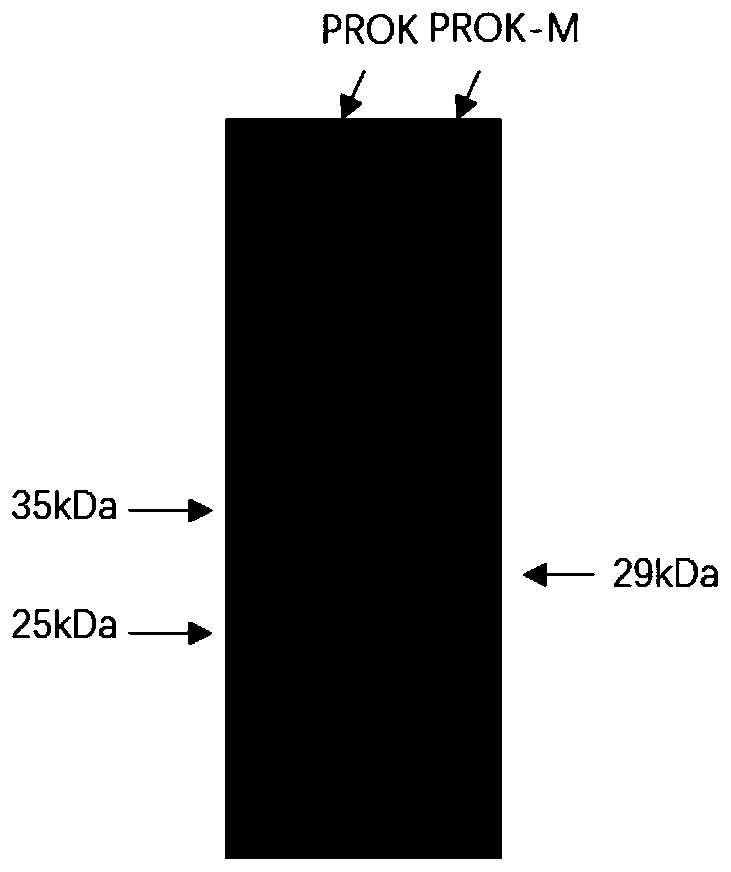
[0058] The supernatant of the recombinant protease expressed in the shake flask was collected, concentrated through a 5kDa membrane bag, and at the same time the medium was replaced with a low-salt buffer, and then f...
Embodiment 3
[0060] The activity analysis of embodiment 3 alkaline protease mutant and wild type
[0061] The activity analysis of the protease of the present invention is carried out by using Folin's phenol reagent chromogenic method. The specific method is as follows: 1 mL of reaction system includes 500 μL of appropriately diluted enzyme solution, 500 μL of substrate, reacted for 20 min under optimal conditions, and added 1 mL of trichloroacetic acid (0.4 mol / L) to terminate the reaction; centrifuged the reaction system at 12000 rpm for 3 min, and aspirated Add 2.5 mL of sodium carbonate (0.4 mol / L) to 500 μL of the supernatant, and then add 500 μL of Folin’s phenol reagent, develop color at 40°C for 20 minutes and then measure the OD value at 680 nm after cooling. Definition of protease activity unit: Under certain conditions, the amount of enzyme required to decompose the substrate casein to generate 1 μmol of tyrosine per minute is 1 activity unit (U).
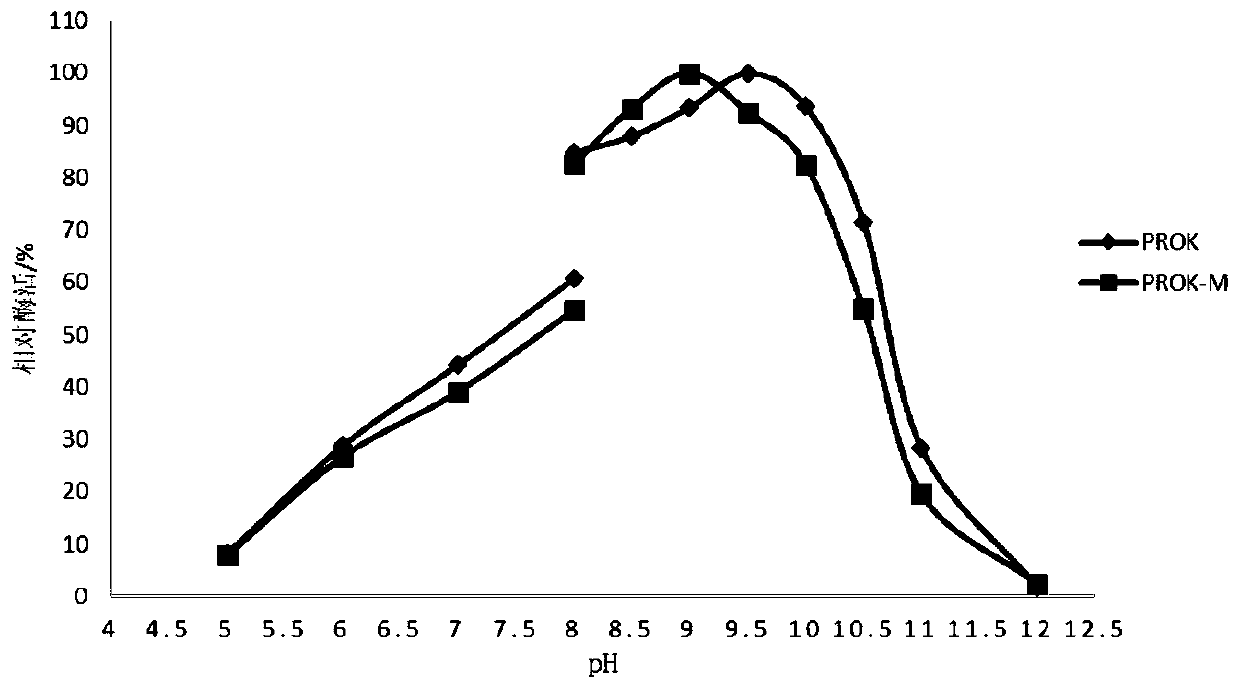
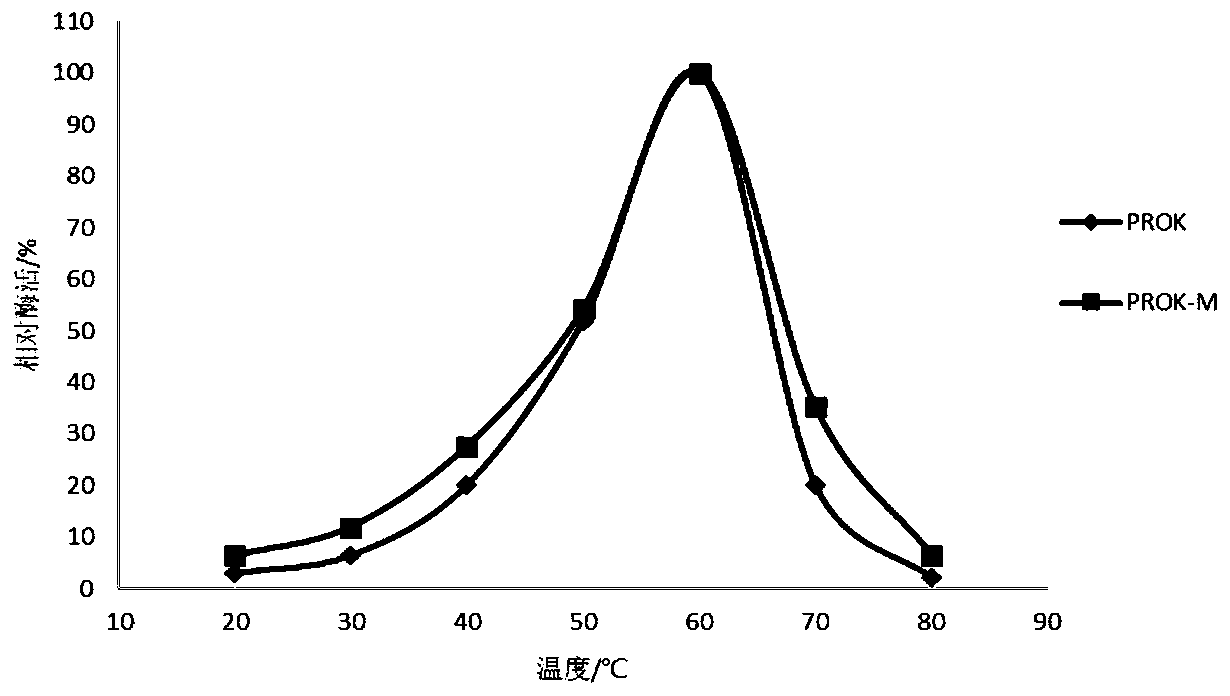
[0062] 1. Comparison of opti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com