Metal@BN core-shell structure nanometer catalyst for synthesis gas methanation reaction, and preparation method thereof
A nano-catalyst and metal nano-particle technology, applied in physical/chemical process catalysts, chemical instruments and methods, and hydrocarbon production from carbon oxides, etc., can solve complex catalyst preparation methods, unsuitable for large-scale preparation, catalyst deactivation, etc. problems, to achieve the effects of avoiding catalyst deactivation, preventing sintering and loss, high and low temperature activity and high temperature stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 1. Preparation of 20wt.% Ni / SiO by impregnation method 2 Catalyst: Dissolve 0.805g of nickel chloride hexahydrate in 4ml of deionized water, then add 2ml of absolute ethanol and stir evenly, then add 0.806g of silica carrier, stir and evaporate to dryness at room temperature; the sample is heated in hydrogen at 450°C Reduction treatment for 2h to obtain 20wt.%Ni / SiO 2 -H 2 Nano catalyst;
[0024] 2. Impregnate 20wt.% Ni / SiO with a boric acid aqueous solution with a concentration of 0.374mol / L 2 -H 2 Catalyst, wherein the B / Ni atomic ratio is 3:1, stirred and volatilized to dryness, and dried at 60°C for 12h; further in NH 3 Nitriding treatment at 850°C for 1 hour in the atmosphere to obtain 20wt.% Ni 1 @(BN) 3 / SiO 2 Core-shell structured nanocatalytic materials.
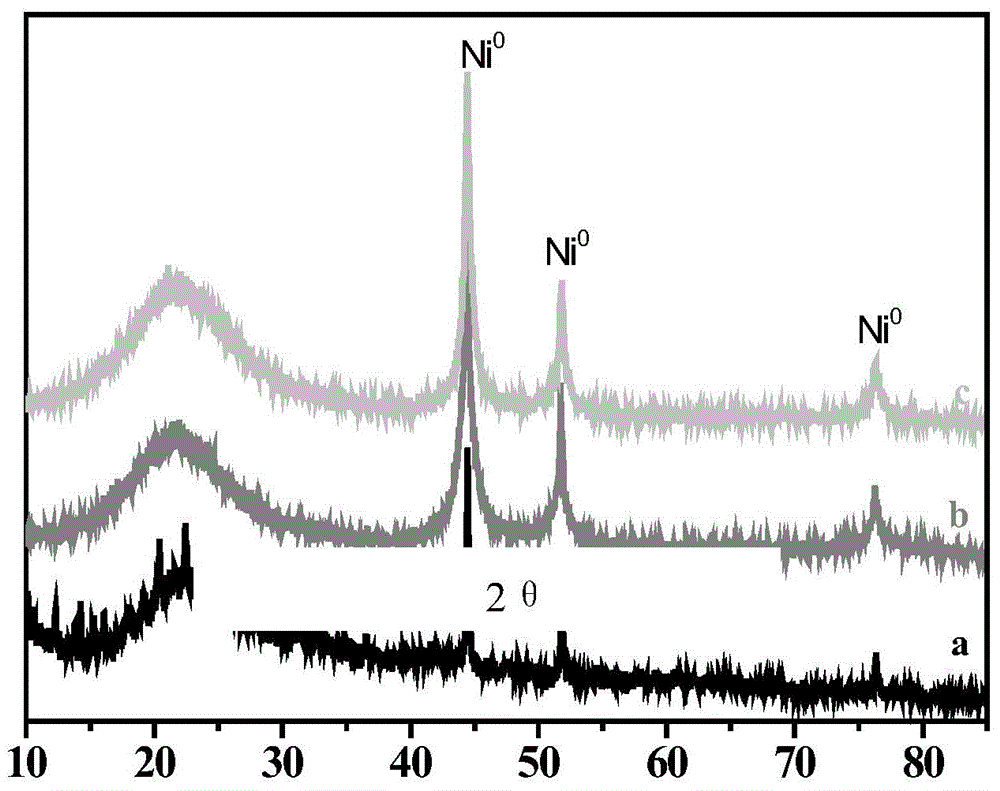
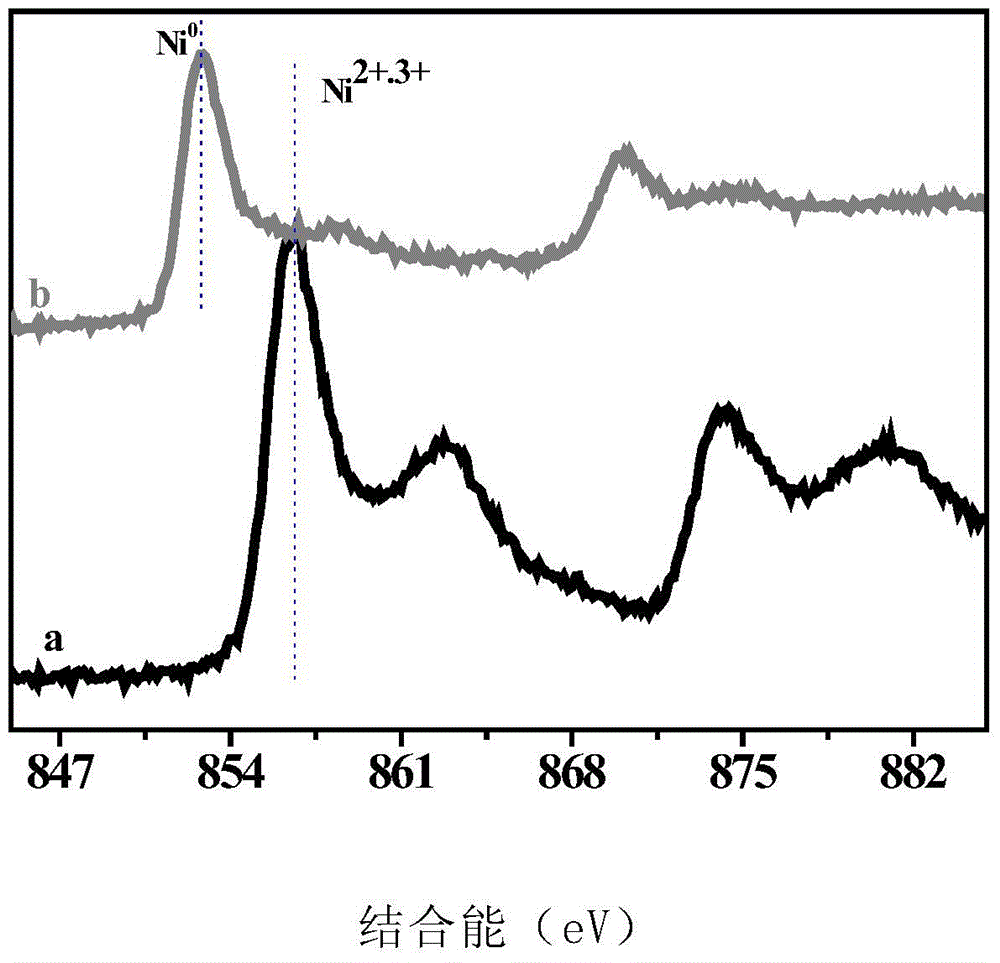
[0025] High-resolution electron microscopy (see figure 1 ) shows that SiO 2 The surface of the loaded Ni nanoparticles is covered by boron nitride to form a core-shell structure. X-ray diffraction ...
Embodiment 2
[0027] 1. Dissolve 0.248g of nickel nitrate hexahydrate in 4ml of deionized water, then add 2ml of absolute ethanol and stir evenly, then add 0.95g of silica carrier, stir and volatilize at room temperature until dry; further put the sample in hydrogen at 650°C Reduction treatment for 2h to obtain 5wt.%Ni / SiO 2 -H 2 Nano catalyst;
[0028] 2. Immerse 5wt.% Ni / SiO with a boron trioxide aqueous solution with a concentration of 0.374mol / L 2 -H 2 Catalyst, wherein the B / Ni atomic ratio is 1:10, stirred and volatilized to dryness, and dried at 60°C for 12h; 2 Nitriding treatment at 750°C for 1 hour in the atmosphere to obtain 5wt.% Ni 10 @(BN) 1 / SiO 2 Core-shell structured nanocatalytic materials.
Embodiment 3
[0030] 1. Dissolve 0.805g of nickel chloride hexahydrate with 4ml of deionized water, then add 2ml of absolute ethanol and stir evenly, then add 0.806g of silica carrier, stir and volatilize to dryness at room temperature, and further place the sample in hydrogen for 500 ℃ reduction treatment for 2h to get 20wt.%Ni / SiO 2 -H 2 Nano catalyst;
[0031] 2. Impregnate 20wt.% Ni / SiO with a boric acid aqueous solution with a concentration of 0.374mol / L 2 -H 2 Catalyst, wherein the B / Ni atomic ratio is 1:1, stirred and volatilized to dryness, dried at 60°C for 12h, and further put the sample in NH 3 Nitriding treatment at 850°C for 1 hour in the atmosphere to obtain 20wt.% Ni 1 @(BN) 1 / SiO 2 Core-shell structured nanocatalytic materials.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com