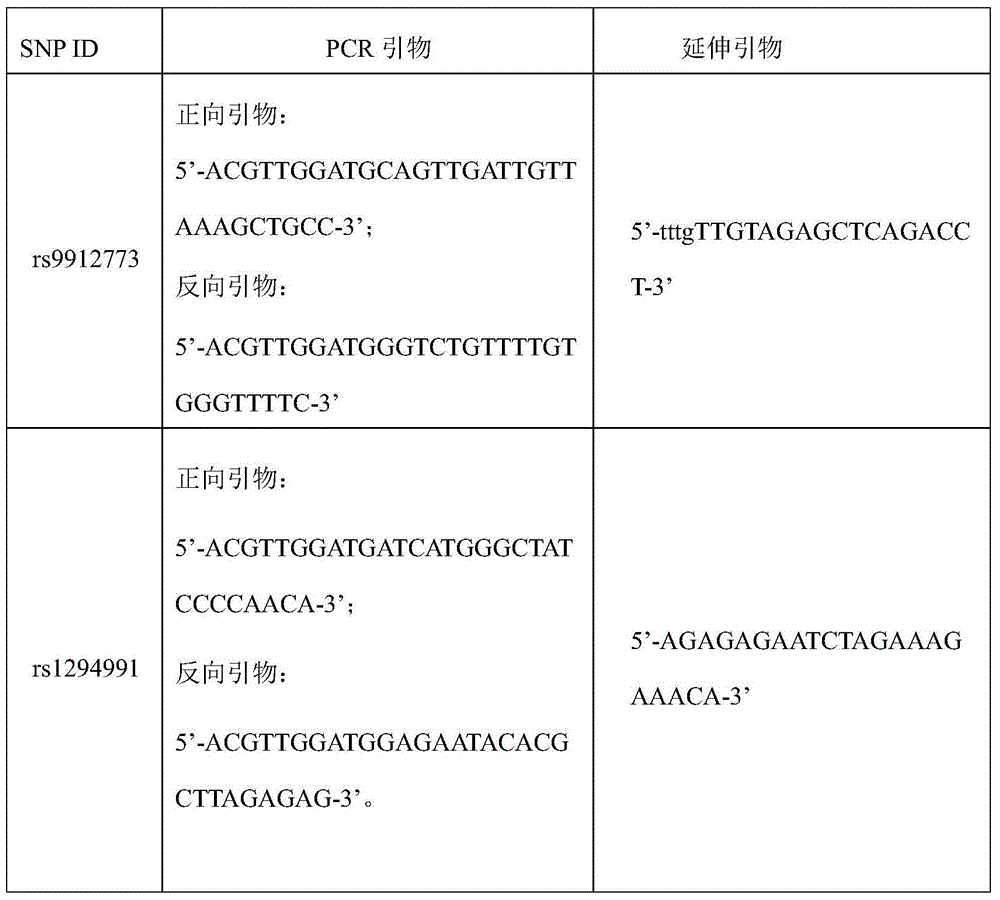
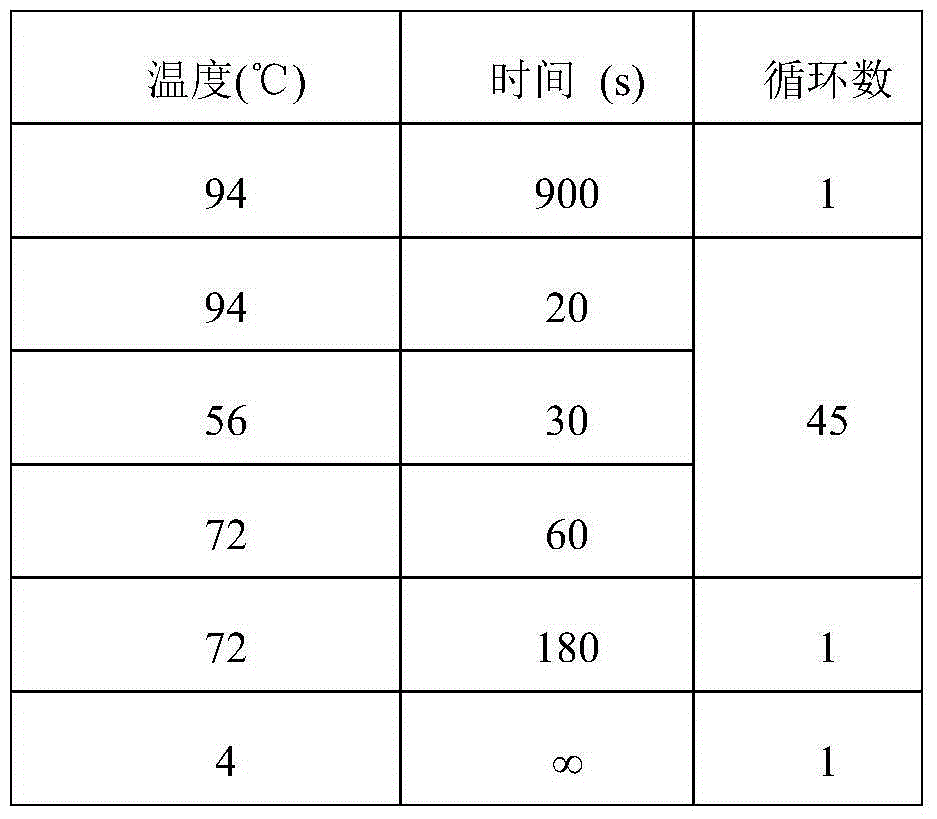
Kit for judging therapeutic response of NSCLC (non-small-cell lung cancer) patient to gefitinib
A technology of gefitinib and a kit, which is applied to a kit for judging the responsiveness of patients with advanced non-small cell lung cancer to gefitinib treatment. The application field of SNP in the preparation of the kit can solve the problem of total effective rate 50%, high detection cost, high equipment requirements, etc., to achieve the effect of facilitating clinical promotion, simple detection process, and low equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0022] specific implementation
[0023] The present invention will be further described below through specific examples. It should be noted that, unless otherwise specified, the examples of the present invention are only used to explain the present invention, and are not meant to limit the protection scope of the present invention.
[0024] 1. Research objects: 86 cases of advanced non-small cell lung cancer. The selected patients (≥18 years old) were confirmed as advanced non-small cell lung cancer by histology or cytology, and all patients provided informed consent.
[0025] 2. Medication method: Gefitinib 250mg, once a day, orally. The medication cycle is 1 year.
[0026] 3. Selection of SNP sites of STAT3 gene: Search the STAT3 gene in the SNP public database (http: / / hapmap:ncbi.nlm.nih.gov / ) provided by the International Human Genome Hapmap Project, and first select the known Han nationality The SNP sites of the information in the population, and then use the tag SNPs ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com