A kind of pharmaceutical composition and its application
A composition and drug technology, applied in the field of traditional Chinese medicine, can solve the problems of patients’ pain and high price, and achieve the effect of good curative effect, high safety and growth inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: The preparation of pharmaceutical composition of the present invention:
[0037] According to the weight ratio between juglandin B and icariin is 1:1, the raw materials are weighed, and the two are mixed evenly to obtain the pharmaceutical composition of the present invention.
[0038] The application of the combined medicine of juglandrin B and icariin, that is, the pharmaceutical composition of the present invention (jugandrin B+icariin) in the treatment of liver cancer will be further described below in conjunction with specific experimental examples.
[0039] 1. Experimental materials:
[0040] 1. Drugs and reagents:
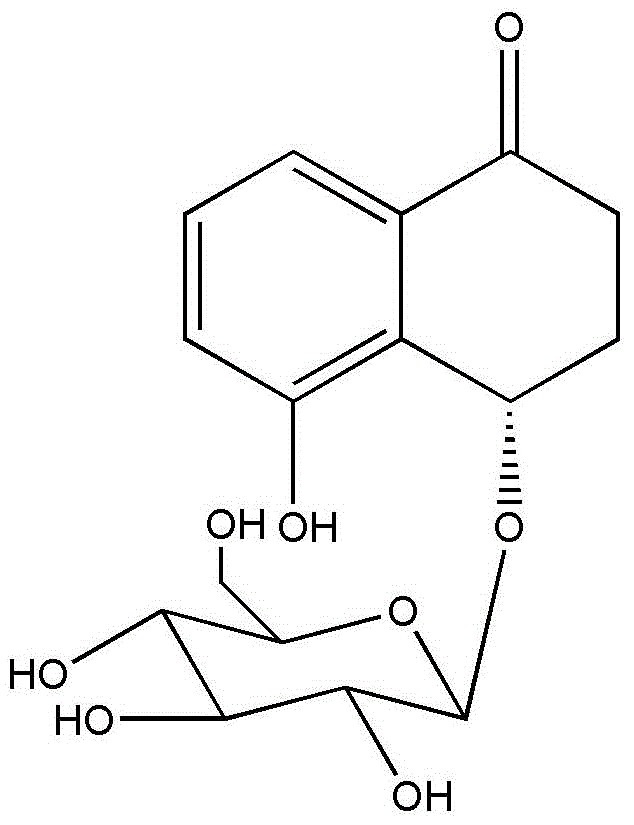
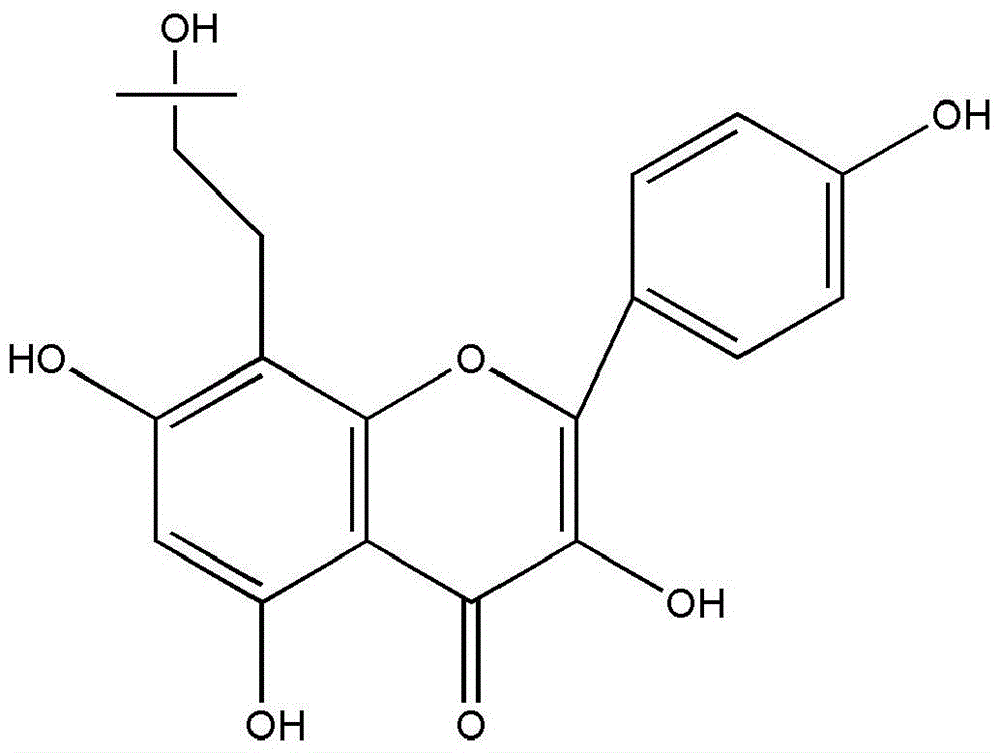
[0041] The extraction method of Juglanin B refers to the article "Isolation of Juglanin B in Beiqinglongyi and Establishment of Content Determination Method" published by Liu Lijuan et al. on pages 46-48 of "Chinese Modern Applied Pharmacy". All icariin was purchased from Shanghai Yuanye Biotechnology Co., Ltd., and the production batch...
experiment example 1
[0049] Experimental example 1: The effect of each administration group on the human liver cancer cell line HepG2 was detected by MTT method:
[0050] The HepG2 cell line was cultured, and when the cells grew to 50-60% confluence, the subjects were divided into 4 groups, namely the Juglandin B group, the Icaritin group, the Juglandin B+Icaritin group and the control group. Correspondingly add 200 μg / mL juglandin B, 200 μg / mL icariin, pharmaceutical composition of the present invention (wherein juglandin B 200 μg / mL+icariin 200 μg / mL) and dissolve in the culture solution of each group 100 μL of DMSO (dimethyl sulfoxide) used for the compound. Cells were cultured for 24 hours, and cell viability was measured using the MTT assay. The method is as follows: remove the supernatant, wash twice with phosphate buffered saline (PBS), incubate with MTT at 37°C for 4 hours, wash twice with PBS, dissolve the crystals with DMSO, measure the absorbance at 490nm, and calculate the growth in...
experiment example 2
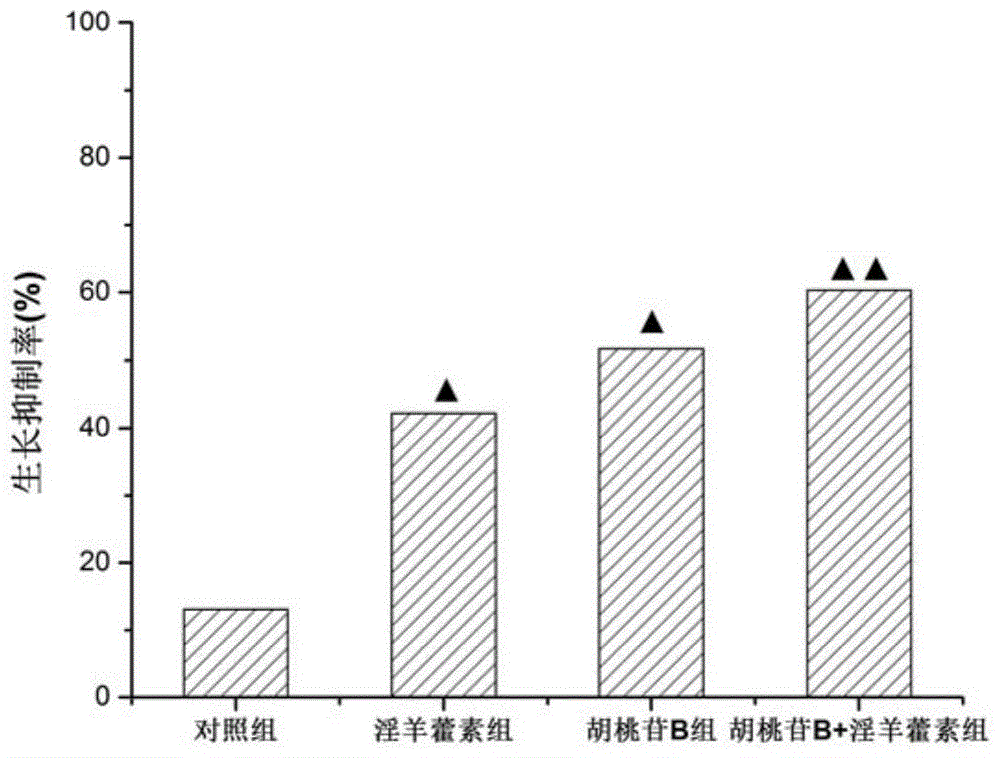
[0056] Experimental example 2: MTT assay was used to detect the effect of each administration group on the mouse hepatoma cell line H 22 Impact:
[0057] culture H 22 Cell line, when the cells grow to 50-60% confluence, the subjects are divided into 4 groups, ie juglandin B group, icariin group, juglandin B+icariin group and control group. Correspondingly add 200 μg / mL juglandin B, 200 μg / mL icariin, pharmaceutical composition of the present invention (wherein juglandin B 200 μg / mL+icariin 200 μg / mL) and dissolve in the culture solution of each group 100 μL of DMSO (dimethyl sulfoxide) used for the compound. Cells were cultured for 24 hours, and cell viability was measured using the MTT assay. The method is as follows: remove the supernatant, wash twice with phosphate buffered saline (PBS), incubate with MTT at 37°C for 4 hours, wash twice with PBS, dissolve the crystals in DMSO, and measure the absorbance at 570 nm.
[0058]
[0059] The experimental results are show...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com