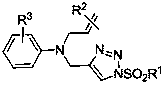
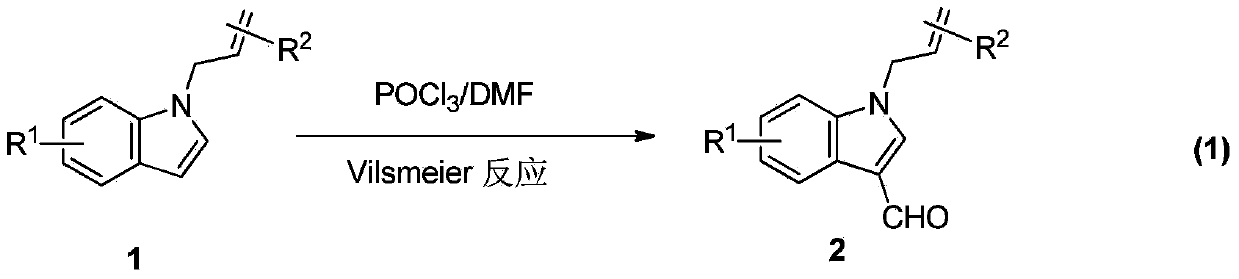
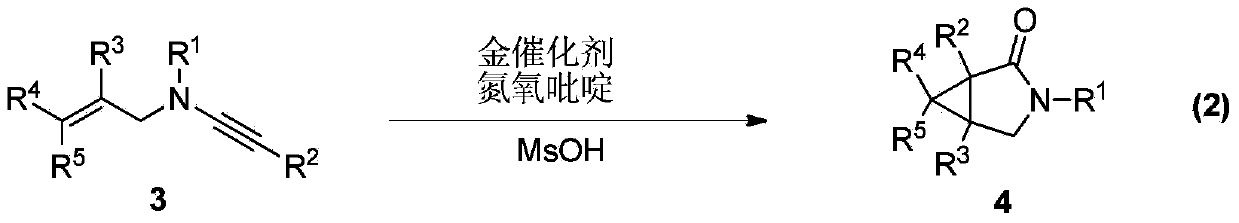
Synthesis method of divergently oriented azacycles
A nitrogen heterocyclic and divergent technology, applied in the field of metal-catalyzed carbene cyclization, can solve the problems of narrow substrate range and difficult preparation of substrates, and achieve the effects of difficult preparation, novel structure and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Mix 2 mmol of 1-p-toluenesulfonyltriazole 7a and 0.01 mmol of rhodium acetate in 10 ml of toluene and stir at a temperature of 120 degrees. After 2 hours, stop heating, add 2 ml of methanol, 5 mmol of potassium carbonate and a drop of water, stirred at room temperature for 12 hours. Extract three times with the organic solvent ethyl acetate, combine the organic phases and wash with saturated brine, then dry with anhydrous sodium sulfate, evaporate the solvent under reduced pressure, use ethyl acetate and petroleum ether as eluents for the residue, and perform silica gel column chromatography After separation and purification, the corresponding nitrogen heterocyclic aldehydes 8a and 9a were obtained (see Table 1). Alternatively, after the reaction is completed, the organic solvent is evaporated under reduced pressure, and the residue is directly separated on a silica gel column.
Embodiment 2
[0034] Mix 2 mmoles of 1-p-toluenesulfonyltriazole 7b and 0.1 mmoles of rhodium acetate in 10 ml of dichloroethane and stir at a temperature of 50 degrees. After 5 hours, stop heating, add 2 ml of methanol, 5 ml Molar potassium carbonate and a drop of water, stirred at room temperature for 8 hours. Extract three times with the organic solvent ethyl acetate, combine the organic phases and wash with saturated brine, then dry with anhydrous sodium sulfate, evaporate the solvent under reduced pressure, use ethyl acetate and petroleum ether as eluents for the residue, and perform silica gel column chromatography After separation and purification, the corresponding nitrogen heterocyclic aldehydes 8b and 9b were obtained (see Table 1). Alternatively, after the reaction is completed, the organic solvent is evaporated under reduced pressure, and the residue is directly separated on a silica gel column.
Embodiment 3
[0036] Mix 2 mmoles of 1-p-toluenesulfonyltriazole 7c and 0.04 mmoles of rhodium acetate in 10 ml of toluene and stir at a temperature of 120°C. After 10 minutes, stop heating and add 2 ml of methanol and 5 mmoles of potassium carbonate. and a drop of water, stirred at room temperature for 12 hours. Extract three times with the organic solvent ethyl acetate, combine the organic phases and wash with saturated brine, then dry with anhydrous sodium sulfate, evaporate the solvent under reduced pressure, use ethyl acetate and petroleum ether as eluents for the residue, and perform silica gel column chromatography After separation and purification, the corresponding nitrogen heterocyclic aldehydes 8c and 9c were obtained (see Table 1). Alternatively, after the reaction is completed, the organic solvent is evaporated under reduced pressure, and the residue is directly separated on a silica gel column.
[0037] Table 1. Preparation of divergent N-allyl-3-indolaldehyde and 3-azabicycl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com