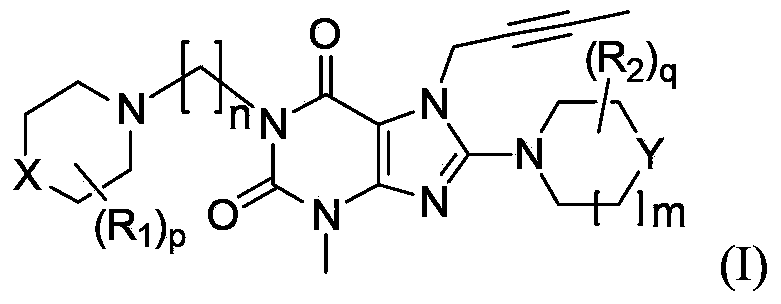
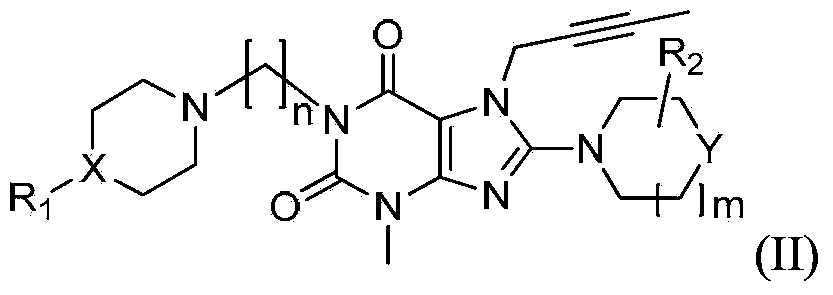
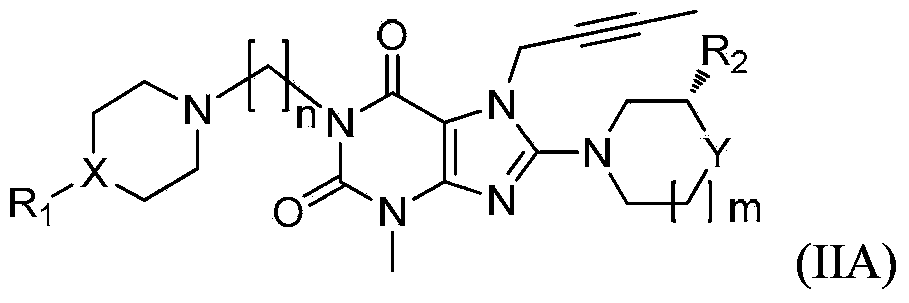
Substituted xanthine compounds, preparation method and applications thereof
A compound, selected technology, applied in the field of medicine, can solve the problem that GLP-1 cannot be made into a drug, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example
[0173] Synthetic route of Scheme1 intermediate a
[0174]
[0175] Preparation of the first step 6-amino-5-nitroso-1-methyluracil 2a
[0176] Dissolve 6-amino-1-methyluracil (10.00g, 70.9mmol) in a mixed solution of 50mL water and 20mL glacial acetic acid, add an aqueous solution of sodium nitrite (7.138g, 103.5mmol) at room temperature 25°C (40 mL), stirred at room temperature for 0.5 h, then raised to 50° C. and stirred for 1 h, and finally moved to room temperature and stirred overnight. After the reaction was detected by TLC, the stirring was stopped, filtered, and the filter cake was washed with water (100 mL) and 95% ethanol (50 mL) to obtain Intermediate 2a, 11.97 g of a purple solid, with a yield of about 99.3%.
[0177] 1 H NMR (DMSO-d 6 ,300MHz)δ:3.166(s,3H,CH 3 ),9.051(s,2H,NH 2 ),11.484(s,1H,NH).MS(ESI + ):m / z=170.3[M+H] +
[0178] Preparation of the second step 5,6-diamino-1-methyluracil 3a
[0179] Dissolve 6-amino-5-nitroso-1-methyluracil 2a (11.50g,...
Embodiment 1
[0222]
[0223] Compound 1 8-[(3R)-3-amino-1-piperidinyl]-7-(2-butynyl)-3,7-dihydro-3-methyl-1-{3-[4- (5-Methylpyrimidine)-1-piperazinyl]propyl}-1H-purine-2,6-dione
[0224]
[0225] 8-[(3R)-3-amino-1-piperidinyl]-7-(2-butynyl)-3,7-dihydro-3-methyl-1-{3-[4-(5 Preparation of -methylpyrimidine)-1-piperazinyl]propyl}-1H-purine-2,6-dione
[0226] Dissolve (R)-3-aminopiperidine 1c (0.050g, 0.50mmol) and potassium carbonate (0.124g, 0.90mmol) in 20mL of anhydrous THF, add 20mg of molecular sieves, and stir at room temperature 25°C for 30min. Gradually add 7-(2-butynyl)-3,7-dihydro-3-methyl-1-{3-[4-(5-methylpyrimidin-2-yl)-1-piperazinyl] Propyl}-1H-purine-2,6-dione b (0.128g, 0.25mmol), heated to 90°C and refluxed for 8h. After the reaction is complete, cool to room temperature 25°C, filter, and concentrate the filtrate under reduced pressure. The obtained crude product is separated by silica gel (300-400 mesh) column chromatography, and the mixture of dichloromethane-methan...
Embodiment 2
[0229]
[0230] Compound 2 8-[(3S)-3-amino-1-piperidinyl]-7-(2-butynyl)-3,7-dihydro-3-methyl-1-{3-[4- (5-Methylpyrimidine)-1-piperazinyl]propyl}-1H-purine-2,6-dione
[0231]
[0232] 8-[(3S)-3-amino-1-piperidinyl]-7-(2-butynyl)-3,7-dihydro-3-methyl-1-{3-[4-(5 Preparation of -methylpyrimidine)-1-piperazinyl]propyl}-1H-purine-2,6-dione
[0233] Using b (0.100g, 0.19mmol) and 2c (0.039g, 0.39mmol) as raw materials, the similar operation steps in Example 1 were used to obtain compound 2, 54 mg of yellow solid, with a yield of 51.9%. m.p.183-184℃;
[0234] 1 H NMR (CD 3 OD,300MHz)δ:1.707(s,3H),1.799(m,2H),1.989(m,2H),2.019(s,3H),2.391(m,4H+2H),3.039(m,2H), 3.162(m,2H),3.372(s,3H),3.584(m,4H),3.710(m,4H),3.969(m,2H),4.909(s,2H),8.068(s,2H).HR -MS(ESI-TOF + ):C 27 h 39 N 10 o 2 Calculated value 535.3252, measured value [M+H] + 535.3244.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com