Preparation method of isopropyl benzene
A technology of cumene and acetophenone, which is applied in the field of hydrogenation of acetophenone into ethylbenzene, which can solve problems such as environmental pollution and poor catalyst stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
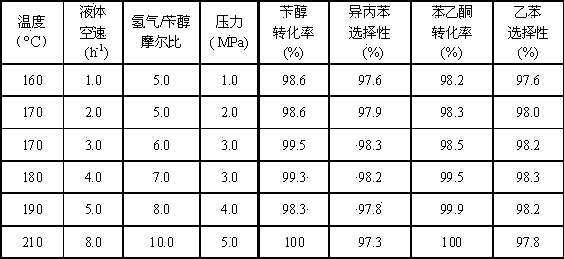
Examples
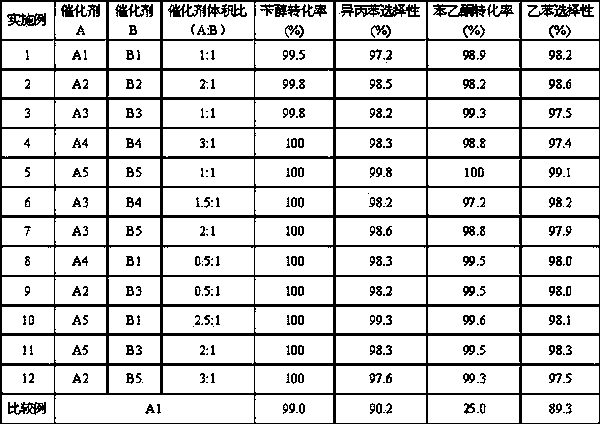
Embodiment 1
[0022] Mix 100.0 g silica with 100.0 g Mg 2+ Immerse in an equal volume of 3% aqueous solution, dry, 500 o After C is roasted, MgO-modified carrier I is obtained, and its composition is: 4.76%MgO-95.24%SiO 2 ; 1.0 g PdCl 2 The solid was dissolved in a solution consisting of 10 g of concentrated hydrochloric acid and 89 g of purified water to obtain solution I. According to the method of equal volume impregnation, 50.0g solution I was impregnated on 50.0g carrier I, and after drying, at 500 o The catalyst was obtained by calcining at C for 4.0 h. The specific composition of catalyst A1 is shown in Table 1.
[0023] Weigh 50.0 g Al 2 o 3 , 10.0 gSiO 2 Carrier, placed in 200 ml of water at 75 o C under stirring to form slurry I; Weigh 150.0 g Cu (NO 3 )2 ?3H 2 O, 50.0 g Zn(NO 3 ) 2 ?6H 2 O, 32.0g Ca(NO 3 ) 2 ?4H 2 O, 30.0 g by weight 50%Mn (NO 3 ) 2 The solutions were dissolved together in 0.8 L of water in Solution I. Add K dropwise to solution I 2 CO 3 aq...
Embodiment 2
[0027] Mix 100.0 g silica with 100.0 g Ca 2+ Immerse in an equal volume of aqueous solution with a content of 6%, dry, 500 o After C is roasted, CaO-modified carrier I is obtained, and its composition is: 7.7%CaO-92.3%SiO 2 ; 0.8 g PdCl 2 The solid was dissolved in a solution consisting of 10 g of concentrated hydrochloric acid and 89.2 g of purified water to obtain solution I. According to the method of equal volume impregnation, 50.0g solution I was impregnated on 50.0g carrier I, and after drying, at 500 o The catalyst A2 was obtained by calcining at C for 4.0 h. The specific composition of catalyst A2 is shown in Table 1.
[0028] Weigh 40.0 g Al 2 o 3 , 20.0 gSiO 2 Carrier, placed in 200 ml of water at 75 o C under stirring to form slurry I; Weigh 200.0 g Cu (NO 3 ) 2 ?3H 2 O, 50.0 g Zn(NO 3 ) 2 ?6H 2 O, 30.0g Mg(NO 3 ) 2 ?6H 2 O, 40.0 g by weight 50%Mn (NO 3 ) 2 The solutions were dissolved together in 0.8 L of water in Solution I. Add K dropwise to ...
Embodiment 3
[0032] Mix 100.0 g silica with 100.0 g Zn 2+ Immerse in an equal volume of aqueous solution with a content of 5%, dry, 600 o After C is roasted, ZnO-modified carrier I is obtained, and its composition is: 5.9%ZnO-94.1%SiO 2 ; 1.5 g PdCl 2 The solid was dissolved in a solution consisting of 10 g of concentrated hydrochloric acid and 88.5 g of purified water to obtain solution I. According to the method of equal volume impregnation, 50.0g solution I is impregnated on 50.0g carrier I, after drying, at 450 o The catalyst A3 was obtained by calcining at C for 4.0 h. The specific composition of catalyst A3 is shown in Table 1.
[0033] Weigh 40.0 g Al 2 o 3 , 30.0 gSiO 2 Carrier, placed in 200 ml of water at 80 o C under stirring to form slurry I; Weigh 150.0 g Cu (NO 3 ) 2 ?3H 2 O, 100.0 g Zn(NO 3 ) 2 ?6H 2 O, 20.0g Zr(NO 3 ) 4 ?5H 2 O, 40.0 g by weight 50%Mn (NO 3 ) 2 The solutions were dissolved together in 0.8 L of water in Solution I. Na was added dropwise ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com