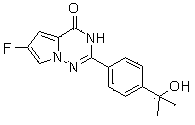
Pyrrolotriazinone derivatives
A pyrrolidinyl compound technology, applied in the field of preparing these compounds, treating diseases, and preparing drugs, achieving good tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0267] Synthesis of 2-p-tolyl-3H-pyrrolo[2,1-f][1,2,4]triazin-4-one ("A1")
[0268]
[0269] Triethylamine (277 μl, 2.00 mmol) was added to a suspension of 1-amino-1H-pyrrole-2-carboxylic acid amide (250 mg, 2.00 mmol) in acetonitrile (4.0 ml). A solution of 4-methylbenzoyl chloride (264 μl, 2.00 mmol) in dichloromethane (0.6 ml) was added dropwise under external cooling with ice. The reaction mixture was stirred at room temperature for 18 hours. The solvent was evaporated and the residue was dissolved in dichloromethane and saturated NaHCO 3 solution. A precipitate formed which was filtered off, washed with water and dried under vacuum to give 1-(4-methyl-benzoylamino)-1H-pyrrole-2-carboxylic acid amide as white crystals; HPLC / MS 1.57 min (A), [M+H] 244;
[0270] 1 H NMR (400 MHz, DMSO-d 6 ) δ [ppm] 11.42 (s, 1H), 7.83 (d, J =8.1, 2H), 7.42 (s, 1H), 7.32 (d, J=8.0, 2H), 6.96 (m, 1H), 6.83 (dd, J =4.2, 1.8, 1H), 6.78 (bs, 1H), 6.08 (dd, J =4.1, 2.8, 1H), 2.38 (s, ...
Embodiment 2
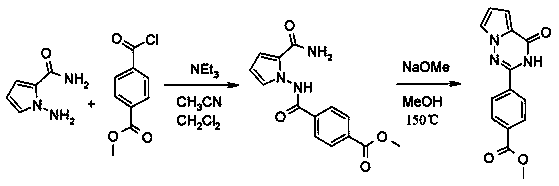
[0274] Synthesis of 4-(4-oxo-3,4-dihydro-pyrrolo[2,1-f][1,2,4]triazin-2-yl)-benzoic acid methyl ester ("A2")
[0275] Triethylamine (554 μl, 4.00 mmol) was added to a suspension of 1-amino-1H-pyrrole-2-carboxylic acid amide (501 mg, 4.00 mmol) in acetonitrile (8.0 ml). Then, a suspension of methyl 4-chlorocarbonylbenzoate (264 μl, 2.00 mmol) in dichloromethane (4.0 ml) was added slowly. The reaction mixture was stirred at room temperature for 18 hours. The solvent was evaporated and the residue was triturated with water. The solid was filtered off, washed with water, and dried under vacuum to give methyl 4-[(2-carbamoylpyrrol-1-yl)carbamoyl]benzoate as white crystals; HPLC / MS 1.45 min (C) , [M+H] 288.
[0276] Sodium (65.3 mg, 2.84 mmol) was dissolved in methanol (5.0 ml). Then, 4-[(2-carbamoylpyrrol-1-yl)carbamoyl]benzoate (544 mg, 1.90 mmol) was added. The mixture was irradiated at 150 °C for 1 h in a microwave reactor. The solvent was evaporated and the residue was ...
Embodiment 3
[0287] 2-[4-(1-Hydroxy-1-methyl-ethyl)-phenyl]-3H-pyrrolo[2,1-f][1,2,4]triazin-4-one ("A5 ")Synthesis
[0288]
[0289] To 4-(4-oxo-3,4-dihydro-pyrrolo[2,1-f][1,2,4]triazin-2-yl)-benzoic acid methyl ester (109 mg, 0.405 mmol) in THF (1.6 ml) was added cerium(III) chloride (110 mg, 0.445 mmol). The mixture was stirred at room temperature for 1 hour. Methylmagnesium chloride (20% solution in THF, 617 μl, 1.70 mmol) was then added and the reaction mixture was stirred at room temperature for another hour. Water was carefully added to the reaction mixture. The mixture was partitioned between 1 N HCl and dichloromethane. The organic phase was dried over sodium sulfate and evaporated. The residue was subjected to silica gel column chromatography using cyclohexane / ethyl acetate as eluent to give 2-[4-(1-hydroxy-1-methyl-ethyl)-phenyl]-3H-pyrrolo[ 2,1-f][1,2,4]triazin-4-one, light brown powder; HPLC / MS 1.58 min (C), [M+H] 270;
[0290] 1 H NMR (400 MHz, DMSO-d 6 ) δ [ppm] 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com