DC (Dendritic Cell) targeting peptide and application of targeting peptide
A technology of targeting peptides and fusion peptides, applied in the field of peptides, can solve the problems of large side effects and unsatisfactory effects of radiotherapy and chemotherapy, and achieve the effect of high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0022] Example one, Using DC cells as target cells to screen and obtain the DC targeting peptide of the present invention
[0023] The present invention uses a phage random peptide library to screen and obtain DC targeting peptides targeting DC cells:
[0024] 1. Screening with DC cells as target cells:
[0025] (1) Obtain DC cells:
[0026] Mononuclear cells (peripheral blood mononuclear cells, PBMCs) were isolated from the peripheral blood of healthy people, and mature dendritic cells (dendritic cells, DCs) were obtained by in vitro induction. Method:
[0027] ① Take fresh peripheral blood from healthy people, add lymphocyte separation medium, and separate by density gradient centrifugation to obtain mononuclear cells.
[0028] ② Cultivate in RPMI-1640 medium containing 10% fetal bovine serum. Place in a 37°C, 5% CO2 incubator for 2 hours and then remove the suspended cells.
[0029] ③ Add rhGM-CSF and rhIL-4 to the culture medium to continue culturing the adherent cel...
example 2
[0041] Example 2, the fusion peptide obtained by fusion of the obtained DC targeting peptide and the tumor-associated antigen MUC1
[0042] 1. Fusion peptide synthesis:
[0043] The screened DC-targeting peptide (phage NP8) was connected to the MUC1 tumor antigen peptide through a flexible linker to synthesize a DC-targeting fusion peptide. At the same time, the FITC-labeled fusion peptide was prepared for use.
[0044]
[0045] 2. Identification of fusion peptide DC binding ability:
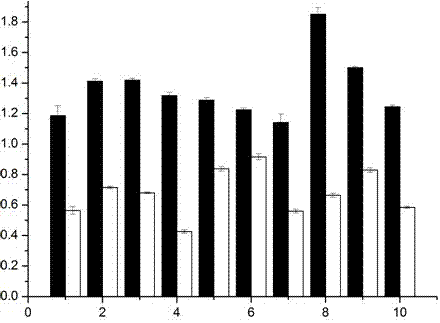
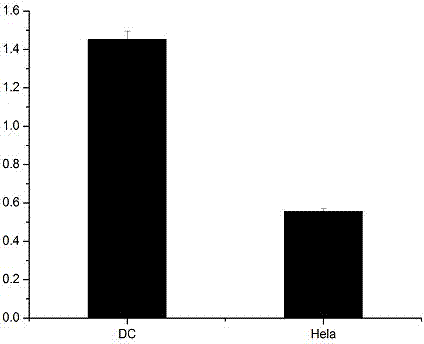
[0046] The prepared NP-MUC1 fusion peptide was identified by the target cell DC, and Hela cells were used as the control. The specific steps were as follows: the DC cells were divided into 1×10 5 The density per well was inoculated on a 96-well plate and placed in a 37°C, 5% CO2 incubator; the cells were subjected to nutrient stress treatment for 2 hours; anhydrous methanol was fixed for 20 minutes, and 0.1% TritonX-100 was treated for 10 minutes; 5% degreasing Block with milk for 1 hour, ad...
example 3
[0049] Example 3, DC fusion peptide effect evaluation
[0050] 1. Uptake of NP-MUC1 Fusion Peptide by Antigen Presenting Cell DC
[0051] We used laser confocal microscopy to observe the efficiency of the fusion peptide into DC cells. The specific steps are as follows: inoculate DC cells in a six-well plate containing small slides and culture in a 37°C, 5% CO2 incubator; then add fluorescein (FITC)-labeled MUC1 polypeptide or FITC-labeled DC fusion peptide. After incubation for 6 hours, the medium was washed away; 10 minutes after 4% paraformaldehyde fixation, 0.1% Triton-x100 was punched for 5 minutes; then DAPI was added for staining, and the antigen-presenting cell DCs were observed by laser confocal microscopy for MUC1 and MUC1 Absorption efficiency of DC targeting fusion peptides.
[0052]
[0053] 2. mixed lymphocyte reaction ( allo - MLR) to measure the antigen presenting ability of antigen presenting cells
[0054] Mononuclear phagocytes and DCs were isolat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com