Dendritic cell targeted peptide, coding gene and application
A technology of dendritic cells and targeting peptides, applied in the direction of peptides, hybrid peptides, specific peptides, etc., can solve the problems of unsatisfactory curative effect, large side effects of radiotherapy and chemotherapy, etc., and achieve improved antigen presentation ability, high safety, The effect of broad application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
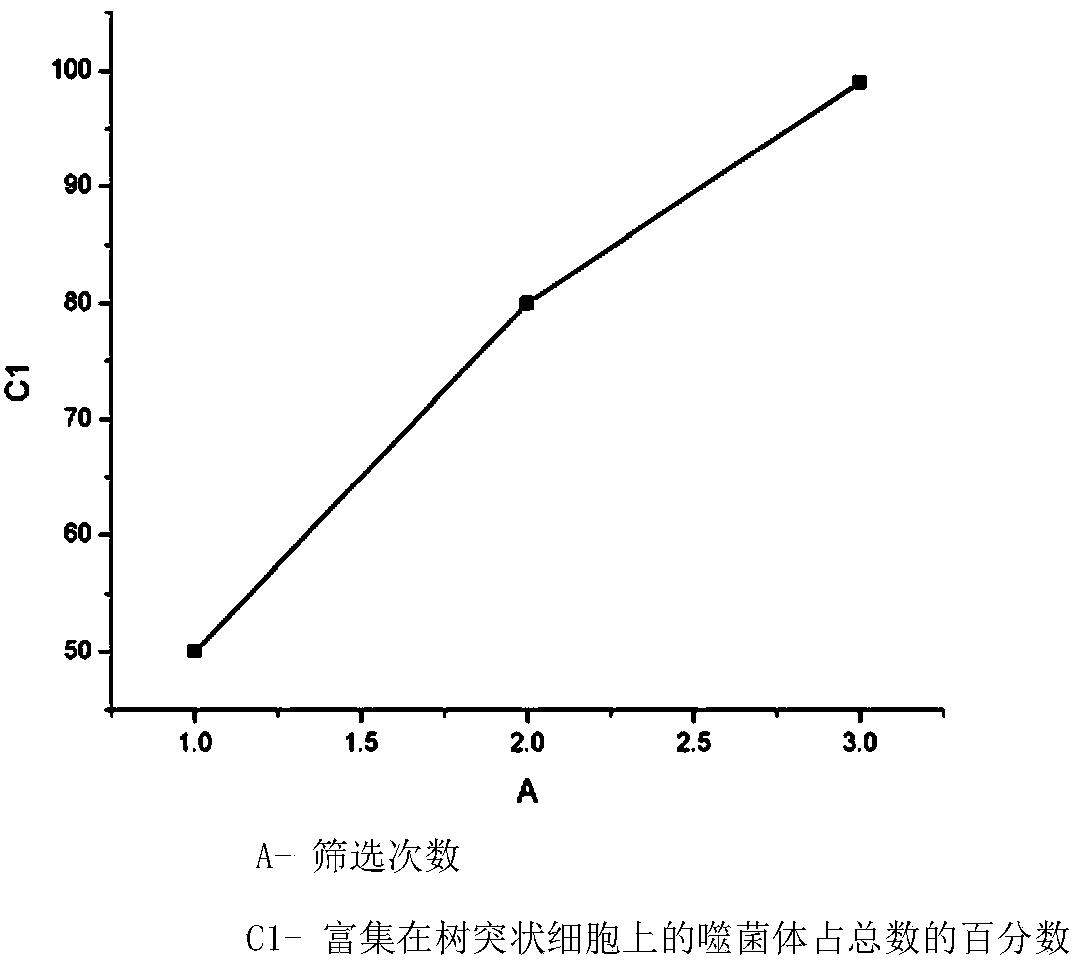
[0034] The dendritic cell-targeting peptide of the present invention is obtained by screening dendritic cells as target cells. The present invention utilizes a commercialized phage display heptapeptide library (New England BioLabs) to screen and obtain the target dendritic cell-targeting peptide. To the peptide:
[0035] 1. Screening with dendritic cells as target cells:
[0036] (1) Obtain dendritic cells:
[0037] Mononuclear cells (peripheral blood mononuclear cells, PBMCs) were isolated from the peripheral blood of healthy people, and mature dendritic cells were obtained by in vitro induction and culture. The method:
[0038] ① Take fresh peripheral blood from healthy people, add lymphocyte separation medium, and separate by density gradient centrifugation to obtain mononuclear cells.
[0039] ②Cultivate in RPMI-1640 medium containing 10% fetal bovine serum. Place at 37°C, 5% CO 2 After standing in the incubator for 2 hours, the suspended cells were removed.
[0040] ③...
Embodiment 2
[0051] Example 2: Obtaining fusion peptides containing dendritic cell targeting peptides
[0052] 1. Synthesis of a fusion peptide (NP-MUC1 for short) containing a dendritic cell targeting peptide:
[0053] The dendritic cell targeting peptide screened in Example 1 is connected to the MUC1 tumor antigen polypeptide through a flexible linker (Cys), and a fusion peptide comprising the dendritic cell targeting peptide is synthesized, such as SEQ ID NO .3 shown. At the same time, prepare the fusion peptide labeled with fluorescein FITC for future use.
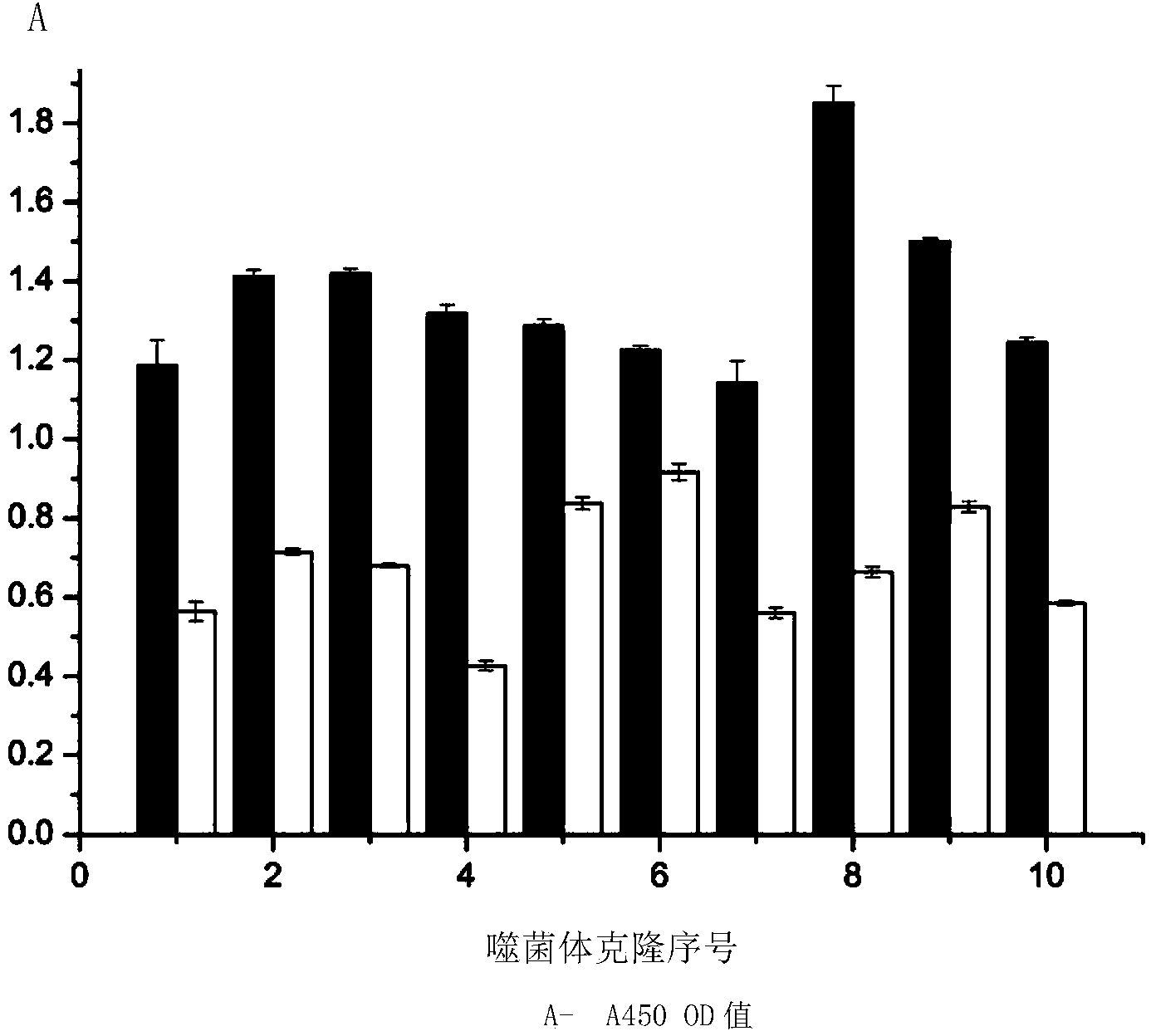
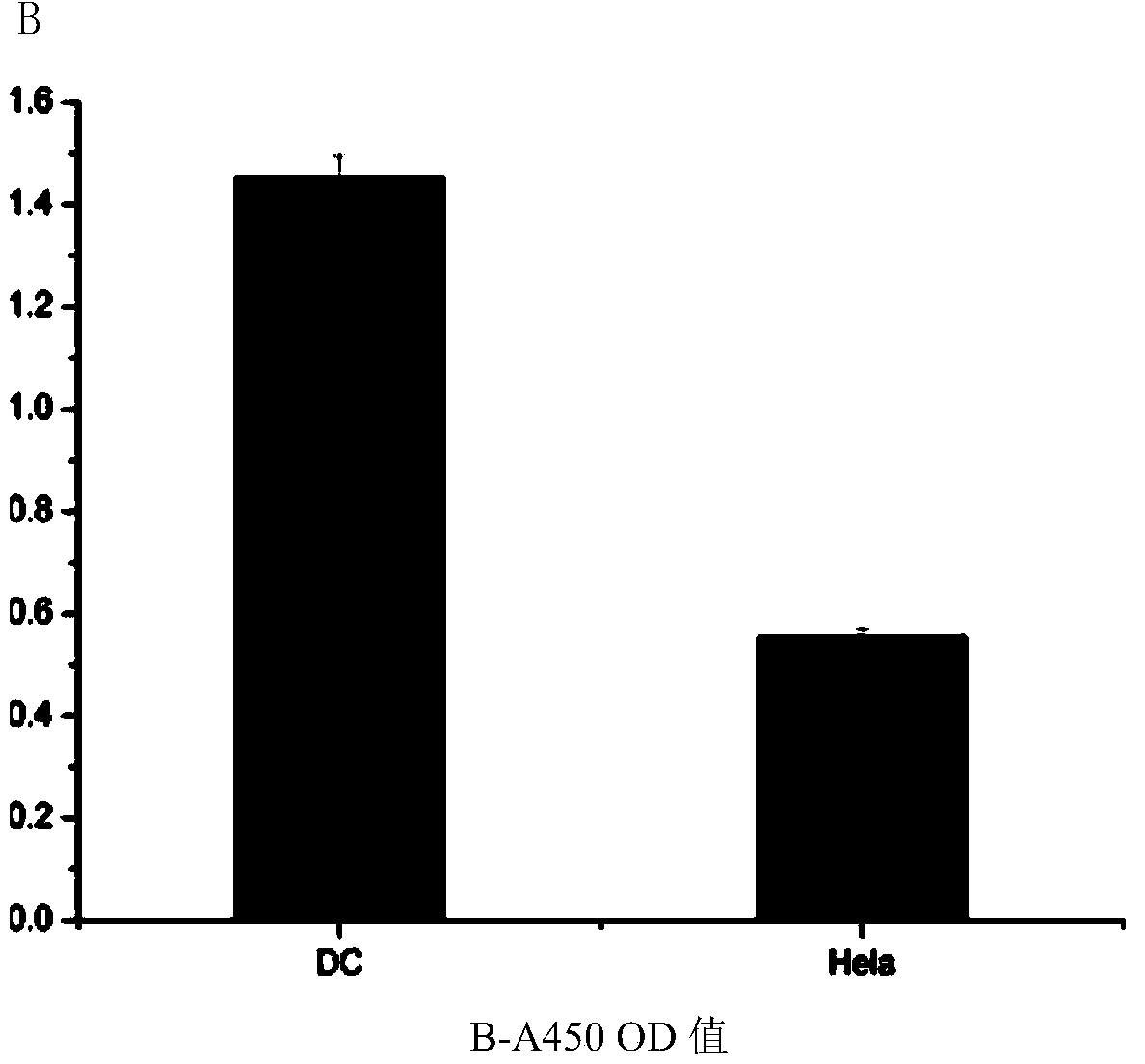
[0054] 2. Identification of the binding ability of the fusion peptide to dendritic cells:
[0055] Dendritic cells were used as the target cells to identify the binding ability of the prepared fusion peptide NP-MUC1, and Hela cells were used as the control. The specific steps are as follows:
[0056] Dendritic cells were divided into 1 × 10 5 Seed on a 96-well plate at a density of 37°C, 5% CO 2 In the incubator; the cells wer...
Embodiment 3
[0058] Example 3: Evaluation of Fusion Peptide Effects
[0059] 1. Absorption of fusion peptide NP-MUC1 by antigen-presenting cells (dendritic cells)
[0060] The efficiency of fusion peptide into dendritic cells was observed by confocal laser microscopy. Specific steps are as follows:
[0061] Dendritic cells were seeded in six-well plates containing slides at 37°C, 5% CO 2 Culture in an incubator; then add fluorescein (FITC)-labeled MUC1 polypeptide or FITC-labeled fusion peptide NP-MUC1. After incubation for 6 hours, wash away the medium; 10 minutes after 4% paraformaldehyde fixation, 0.1% Triton-x100 for 5 minutes; then add DAPI staining, use laser confocal microscope to observe antigen-presenting cells for MUC1 polypeptide and Uptake efficiency of targeting fusion peptide NP-MUC1. Experimental results such as Figure 4 As shown, the results indicated that the uptake efficiency of the fusion peptide NP-MUC1 by antigen-presenting cells was significantly higher than tha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com