Allium fistulosum leaf agglutinin rcombinant protein, its encoding polynucleotide, primer and process for preparation thereof
一种重组蛋白、凝集素的技术,应用在葱叶凝集素的核酸序列编码领域,能够解决破坏膜完整性、昆虫摄取食物或吸收营养物质的能力降低、破坏组织完整性等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1. Isolation and purification of proteins
[0055] Onion leaves were collected from the National Institute of Plant Genetic Resources at Regional Centre, Bhawali, Uttaranchal, India. Its protein was purified according to a published protocol (Smeets et al., 1997b). The purified protein was further purified using a 50 kDa cut-off filter unit. Purified proteins were pooled on 10 kDa cut-off filters and stabilized in phosphate buffered saline (PBS) for experiments.
Embodiment 2
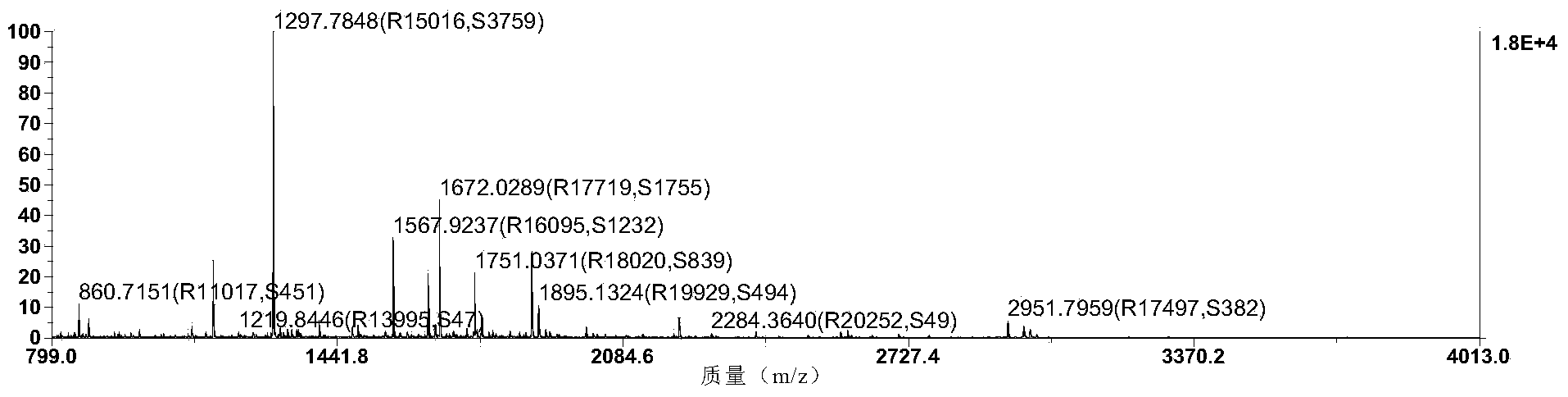
[0056] Example 2. Peptide Mass Fingerprinting Method
[0057] The purified protein was resolved by SDS-PAGE. Protein bands were excised and trypsinized prior to peptide mass fingerprinting. Bioinformatics analysis (MASCOT search) was performed on the data. Its peptide was found to match the mannose-binding lectin ( figure 2 ).
Embodiment 3
[0058] Example 3. Hemagglutination test
[0059] Hemagglutination tests were performed using rabbit erythrocytes in V-bottom microtitre plates. The total volume tested was 100 μl, and 50 μl aliquots of 2-fold serial dilutions of the lectin in PBS were mixed with 50 μl of a 2% trypsinized suspension of rabbit erythrocytes. The microplate was incubated for 1 hour at room temperature. Agglutination was assessed visually. The titer of cell agglutination was taken as the reciprocal of the maximum dilution of the lectin with measurable agglutination.
[0060] Table 1. Hemagglutination tests for AFAL and their comparison with ASAL
[0061] S.N.
[0062] 2
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com